Abstract
Introduction
Prevention of the rapid growth in incidence of type 2 diabetes (T2DM) is a big challenge for clinicians. In China, many trials have indicated that Tianqi capsule, which contains several Chinese herbal medicines as part of a large healing system called traditional Chinese medicine, could decrease the incidence of T2DM. The review assessed the effectiveness of Tianqi capsule in prevention of T2DM.
Methods
Seven electronic databases were searched to identify eligible trials published from the inception of the databases up until May 1, 2017. Randomized controlled trials (RCTs) of Tianqi capsule for impaired glucose tolerance (IGT) were included. Data extraction and quality assessment were performed according to the Cochrane review standards. A random or a fixed effect model was used to analyze outcomes which were expressed as risk ratios (RRs) or mean differences (MD), and I 2 statistics were used to assess heterogeneity.
Results
Six trials were identified that included 1027 subjects. Meta-analysis showed that subjects who received Tianqi capsule plus lifestyle modification (LM) were less likely to progress to T2DM compared to controls (RR 0.55, 95% CI 0.44–0.68). Subjects who received Tianqi capsule plus LM were more likely to have glucose return to normal compared to controls (RR 0.69; 95% CI 0.62–0.78); and they had reduced fasting plasma glucose (FBG) (MD − 0.35; 95% CI − 0.55 to − 0.16) and 2-h plasma glucose (2 h PG) (MD − 1.04; 95% CI − 1.75 to − 0.32). There was no statistical difference between the two groups for IGT stabilized incidence (RR 0.89; 95% CI 0.71–1.12). No obvious adverse events occurred.
Conclusion
In patients with IGT, Tianqi capsule reduced the risk of progression to T2DM and increased the possibility of regression toward normoglycemia. As a result of the limited number of RCTs and the methodological drawbacks of the included studies, the results should be interpreted with caution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prediabetes was defined in 2015 by the American Diabetes Association (ADA) as including impaired fasting glucose (IFG) or impaired glucose tolerance (IGT), or mild elevation of hemoglobin A1c (HbA1c) in the 5.7–6.4% range, and indicated an increased risk of progression to diabetes and the development of cardiovascular events [1]. The prevalence of prediabetes is 50.1%, accounting for a total of 493.4 million people in China [2], and 70.7% of those with prediabetes had IGT [3] and form an important high-risk target group for intervention aimed at preventing T2DM [4, 5]. Current guidelines recommend that IGT patients undergo lifestyle modifications, which have been shown to be effective for reducing the incidence of T2DM [6,7,8]. However, it is not easy to maintain rigorous and sustained lifestyle intervention for the long term. Metformin is commonly used to treat prediabetes and acarbose use is restricted to specific countries, but neither is formally approved for this indication, while other oral anti-diabetic drugs and anti-obesity drugs are also not currently recommended for diabetes prevention, although randomized controlled trials (RCTs) have shown effectiveness [9, 10].
Chinese herbs have been applied to treat diabetes, and a few are suggested to be modestly useful. For instance, Tianqi capsule is reported to decrease hemoglobin A1c and blood glucose [11,12,13,14]. It consists of Radix Astragali (Huang Qi), Radix Trichosanthis (Tian Hua Fen), Rhizoma Coptidis (Huang Lian), Radix et Rhizoma Ginseng (Ren Shen), Caulis Dendrobii (Shi Hu), Herba Ecliptae (Mo Han Lian), Cortex Lycii (Di Gu Pi), Fructus Ligustri Lucidi (Nv Zen Zi), and Fructus Corni (Shan Zhu Yu). Clinical studies and animal experiments have also demonstrated that Tianqi capsule could reduce the risk of T2DM and increased the regression towards normoglycemia, but systematic evidence is lacking. We performed a systematic review and meta-analysis to assess the efficacy and safety of Tianqi capsule in preventing T2DM.
Methods
The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration no. CRD 42017068571: http://www.crd.york.ac.uk/PROSPERO/myprospero.php). This article was written using PRISMA reporting guidelines and was based on previously conducted studies. These data do not include any new human or animal studies performed by the authors.
Search Strategy
The systematic review and meta-analysis was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions. Trials were identified from the Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PubMed, EMBASE, Chinese Biomedical Database (CBM), Chinese National Knowledge Infrastructure (CNKI), and the Wanfang database. Search terms (free word search) were as follows: (“Tianqi capsule” OR “Tian Qi” OR “Tian qi”) AND (“impaired glucose tolerance” OR “prediabetes” OR “pre-diabetes” OR “IGT” OR “hyperglycemia” OR “diabetes” OR “prevention”) AND (“randomized controlled trial” OR “controlled clinical trial” OR “random” OR “randomly” OR “randomized” OR “control”). We searched for all relevant articles published from inception of each database until May 1, 2017, and we restricted our search to trials published in Chinese and English. We contacted authors of original studies for additional data when necessary.
Selection Criteria
We restricted the analysis to RCTs that compared Tianqi capsule with controls. We assessed treatment groups and did not restrict controls, which included placebo, no treatment (blanks), or Western medication. We included IGT patients irrespective of gender, age, and ethnicity, but all patients were diagnosed with IGT by clearly defined or internationally recognized criteria. Studies were excluded if they used interventions combined with other kinds of traditional Chinese medicine (TCM) therapies except for Tianqi capsule (acupuncture, acupoint injection, other herbal formulas to avoid confounding information). Non-randomized trials were excluded.
Outcome Measures
We assessed the incidence of diabetes and regression towards normoglycemia as primary outcomes. The incidence of T2DM refers to the number of participants who had progressed to T2DM according to World Health Organization (WHO) or ADA criteria [fasting plasma glucose (FPG) ≥ 7.0 mmol/L, or 2-h postprandial blood glucose (2 h PG) ≥ 11.1 mmol/L] by the end of the trial. Regression towards normoglycemia refers to the number of participants who returned to normal blood glucose range [FPG < 6.1 mmol/L, or 2 h PG < 7.8 mmol/L] by the end of the trial. Secondary outcomes were as follows: (1) IGT stabilized incidence, which refers to the number of participants who still had IFG (FBG 6.1–6.9 mmol/L and 2 h PG < 7.8 mmol/L) and/or IGT (FBG < 7.0 mmol/L and 2 h PG 7.8–11.0 mmol/L) by the end of the trial; (2) glycemic control based on FBG and 2-h postprandial blood glucose; (3) body mass index (BMI), which is defined as the body weight divided by the square of the body height; (4) adverse events.
Data Extraction and Quality Assessment
Two reviewers extracted data independently using a predesigned collection form to collect title, authors, year; study population size, age, gender; intervention dose and frequency; details of controls; and course of treatment, follow-up length, outcome measures, and adverse events. Discrepancies were settled by consensus or a third party.
Two reviewers independently assessed methodological quality of the RCTs using a Cochrane Collaboration Risk of Bias tool. Risk of bias was assessed according to the Cochrane Handbook [15, 16] and consists of random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessment; incomplete outcome data; and selective reporting and other sources of bias. Each item was categorized “high risk” (at least one item had a high risk of bias), “low risk” (all items had a low risk of bias), or “unclear” (at least one item had an unclear risk of bias). Other bias included a profit bias, and the placebo effect could not be ruled out. Discrepancies in this interpretation were resolved by consensus or a third party.
Assessment of Evidence Quality
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to assess the quality of the evidence for each outcome. According to GRADE, outcomes of an intervention are categorized into four levels of evidence quality: + very low, ++ low, +++ moderate, and ++++ high. Using GRADE, we addressed the risk of bias (in individual studies), inconsistency (heterogeneity in estimates of effect across studies), indirectness (related to the question or due to intransitivity), imprecision, and publication bias. RCTs are considered higher-quality evidence, and observational studies are of lower quality so we applied these criteria to summarize the evidence [17].
Data Synthesis
We combined trials using Rev Man 5.3 software (Cochrane Collaboration, Oxford, UK). For dichotomous outcomes, we calculated risk ratios (RRs) using the Mantel–Haenszel method, and for continuous variables, we calculated weighted mean differences (MDs) using the inverse variance method. ITT (intention-to-treat) analysis was applied to the data. For all estimates, we computed the 95% confidence intervals (CIs). We quantified statistical heterogeneity using the I-squared statistic (I 2), statistical heterogeneity between the trials was significant when I 2 > 50%. A fixed-effects (FE) model was used if there was no significant data heterogeneity (I 2 < 50%); and a random-effects (RE) model was used if significant heterogeneity existed. Publication bias was assessed through visual inspection of funnel plots. Sensitivity and subgroup analyses were done to assess robustness of data for primary outcomes.
Results
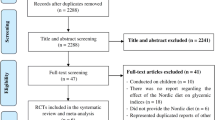
A PRISMA flow diagram of this review is depicted in Fig. 1 and study characteristics are summarized in Table 1. WHO criteria were used to assess IGT [22, 23]. An overview of the judgments regarding each risk of bias items in the included trials is shown in Fig. 2. Four trials reported random sequence generation; two trials reported using random allocation, and one gave no information about allocation concealment. Five trials reported blinding of participants and personnel. All included trials provided complete baseline information and described similarities between groups compared. Three trials reported dropouts or withdrawals [18,19,20,21,22,23]. One trial [18] was at low risk of selective reporting bias due to trial protocols being available.
Study Characteristics
The basic characteristics of these studies are summarized in Table 1. A total of 1027 participants were involved (526 in the treatment group and 501 in the control group). All trials involved recruited patients or outpatients. Trial sample size ranged from 60 to 420 participants, and 498 male patients and 529 female patients were included. The mean age was 51.5 years (range 25–70 years), and the mean BMI was 25.0 kg/m2. All included studies enrolled participants suffering from IGT, which were diagnosed with IGT according to WHO DM criteria (1999). Wei and Wang [22, 23] mentioned that the pattern of the syndrome was consistent with dual deficiency of Qi-yin and heat, while the remaining four trials did not classify the syndrome according to traditional Chinese medical theory. Five trials compared Tianqi capsule plus lifestyle modification (LM) with placebo plus LM, and one trial compared Tianqi capsule plus LM with LM alone. The intervention period lasted from 6 to 24 months. All of the trials reported the incidence of T2DM, normalization of blood glucose, and IGT stabilized incidence, while five trials [19,20,21,22,23] reported the changes in the FPG and/or 2-h plasma glucose levels, and three trials [18, 20, 22] reported BMI.
Methodological Quality Assessment
An overview of the judgments regarding each risk of bias item in the included trials is shown in Fig. 2. Four trials reported the method of random sequence generation, whereas the other two trials reported “randomly allocating”, but the detailed method of randomization was not provided. Two trials reported the method of allocation concealment, and five trials reported the blinding of participants and personnel. All the included trials provided completed baseline information and described similarities between comparison groups, and three trials reported dropouts or withdrawals. Furthermore, we judged one trial to be at low risk of selective reporting bias because their trial protocols were available.
Primary Outcomes
Incidence of Diabetes
Six trials reported on the incidence of T2DM in the groups, and these trials showed insignificant heterogeneity of the trial results (I 2 = 0%); thus, a fixed-effects model was used for statistical analysis. Meta-analysis showed that there was a significant difference found in favor of the treatment group (n = 1027, RR 0.55; 95% CI 0.44–0.68) (Fig. 3).
Regression Towards Normoglycemia
Six trials reported on the regression to normal blood glucose in the groups, and these trials showed insignificant heterogeneity of the trial results (I 2 = 0%); thus, a fixed-effects model was used for statistical analysis. The results showed that those receiving Tianqi capsule plus LM combination were more likely to have normalized the blood glucose compared to controls (n = 1027, RR 0.69; 95% CI 0.62–0.78) (Fig. 4).
Secondary Outcomes
IGT Stabilized Incidence
Six trials had insignificant heterogeneity for IGT stabilization (I 2 = 43%). Thus, a fixed-effects model was used for statistical analysis and there were no statistical differences between either group (n = 1027, RR 0.89; 95% CI 0.71–1.12). IGT stabilized incidence was similar between treatment and control groups (Fig. 5).
Fasting Blood Glucose and Two-Hour Postprandial Blood Glucose
Five trials used FPG as an outcome, and heterogeneity between trials was insignificant (I 2 = 63%); therefore, a random-effects model was used for statistical analysis. A pooled analysis showed a statistically significant decrease in FPG with Tianqi capsule + LM compared to controls (n = 607, MD − 0.35; 95% CI − 0.55 to − 0.16). In five trials, data for 2 h PG variations were measured, and heterogeneity between trials was significant (I 2 = 77%); therefore, a random-effects model was used for statistical analysis. The results showed that there was a statistically significant difference between two groups for 2 h PG (n = 607, MD − 1.04; 95% CI − 1.75 to − 0.32) (Figs. 6 and 7).
Body Mass Index
BMI data from three trials appear in Fig. 8 and comparisons of BMI with Tianqi capsule and LM with controls showed heterogeneity between trials (I 2 = 96%). Thus, a random-effects model was used for statistical analysis and there was no statistical difference between two groups for BMI variations (n = 617, MD − 0.04; 95% CI − 1.94 to 1.85) (Fig. 8).
Adverse Events
Adverse effects were reported in four trials and two reported no adverse events (Table 2) [18, 22]. The results showed that there were no significant differences between these two groups (n = 420, RR 1.18; 95% CI 0.58–2.40). There were also no statistical differences between groups or in routine blood, urine, liver and renal functions studies, or ECG data before and after treatment. Sun’s group [19] showed that some people developed mild gastrointestinal symptoms during early stages of taking Tianqi capsule and that this resolved. Wang’s group [20] reported that the liver function of IGT patients did not change obviously after the treatment of Tianqi capsule, but the level of serum creatinine was significantly decreased in the two groups compared with before the treatment (P < 0.05).
Sensitivity Analysis
To examine the stability of the results, a sensitivity analysis of incident diabetes was performed to investigate the influence of missing data. The analysis included two kinds of scenario: (1) Extreme case analysis favoring Tianqi capsule + LM (“best–worst” case scenario): none of the dropouts/participants lost from the treatment group, but all of the dropouts/participants lost from the control group experienced the outcome. (2) Extreme case analysis favoring placebo + LM (“worst–best” case scenario): all dropouts/participants lost from the treatment group, but none from the control group experienced the outcome. Given that the results of the two methods were inconsistent (best–worst: RR 0.48; 95% CI 0.39–0.59; worst–best: RR 0.65; 95% CI 0.53–0.80), the consistency of results further supported the credibility of the meta-analysis. The results are shown in Fig. 9.
Subgroup Analysis
Post hoc sensitivity analysis was done via a limiting meta-analysis to assess treatment duration, sample size, placebo effects, and publication language. There was much overlap and little difference in confidence intervals and in overall risk ratios for incident diabetes and glucose normalization (Tables 3 and 4). All I 2 values were less than 50% and most were 0%, indicating a low heterogeneity. There was no significant difference (P > 0.05) in overall risk ratios for all subgroup analysis.
Publication Bias
Publication bias was assessed by using funnel plots. The funnel shape of the plot was not completely symmetrical, indicating a potential publication bias (Fig. 10a, b).
Assessment of Quality of Evidence
Figure 11 showed overall evidence quality for each outcome (except adverse events) using the GRADE method. Generally, evidence quality was moderate for Tianqi capsule and diabetes, and normalization of blood glucose and stabilized IGT; however, evidence was poor for Tianqi capsule and FBG, 2 h PG, and BMI.
Discussion
Summary of Evidence and Explanation of Results
Six RCTs with 1027 participants were included in the meta-analysis and trials were homogeneous with respect to dose and criteria used to define progression or regression of diabetes. These six trials covered urban, rural, village, and populations. The results indicated that Tianqi capsule could decrease the risk of progression to T2DM and increase the regression towards normoglycemia (Figs. 3 and 4). The pooled estimates changed little in the sensitivity analysis, which further supported the credibility of the meta-analysis (Fig. 8). Additionally, Tianqi capsules were associated with significantly decreased FBG and 2 h PG (Figs. 6 and 7). There was no statistical difference regarding adverse events that happened between the Tianqi group and the control group (Table 2). Merlotti et al. published a systematic review and meta-analysis assessing strategies for preventing diabetes [10]; the results showed that 12 kinds of strategies were useful for preventing diabetes and these had different efficacies [from RR 0.37 (95% CI 0.26–0.52) to 0.85 (95% CI 0.77–0.93)]. The effect of Tianqi capsule on incidence of diabetes was similar to that of α-glucosidase inhibitors (RR 0.54; 95% CI 0.39–0.75) and physical activity alone (RR 0.53; 95% CI 0.40–0.70).
Our results agree with those of Grant et al. [24], whose study included both IGT and IFG patients, suggesting that Chinese herbal medicine and LM was more effective for reducing the incidence of diabetes compared to LM alone. Also, Chinese herbal medicine and LM was more than twice as likely to normalize FBG compared to LM alone. The strengths of our review were that, firstly, we included studies that were of better quality according to Jadad scores, and most included were blinded and used a placebo; we also focused on IGT, which decreased heterogeneity.
Previous studies of 300 cases showed that Tianqi capsule decreased HbA1c by 1.15 ± 1.58% after 8 weeks of treatment [13]. Proteomics showed that the capsule modified glucolipid metabolism by upregulating transthyretin (TTR), haptoglobin (Hp), serum amyloid p-component (SAP), and prothrombin, and downregulating apolipoprotein E, apolipoprotein A-I, and Ig gamma-2A chain C region [25]. Metabonomics demonstrated that Tianqi capsule regulated lipid metabolism and improved insulin resistance [26].
IGT can lead to diabetes which can increase the risk of macrovascular and microvascular diseases. Therefore, early intervention for IGT is needed and prevention of diabetes is desirable. In a diabetes prevention programme (DPP) study, moderate lifestyle intervention reduced relative risk of diabetes by 28% [27], and there was a 41% reduction in diabetes during a 6-year follow-up in a second study [6]. One study suggested that Tianqi capsule combined with moderate lifestyle modification reduced diabetes risk by 32.1% [18]. Compared to international large-scale clinical trials of lifestyle modification for diabetes prevention, Tianqi capsule showed preferable effectiveness, whilst avoiding the rigorous and sustained lifestyle intervention that could not be tolerated, and patients showed good compliance. In the drug intervention study, acarbose was shown to reduce the risk of diabetes by 25% (STOP-NIDDM) [28], but this drug is not widely available or approved in the USA. Metformin can reduce the risk of diabetes by 31% (American DPP) [8] and this drug has widespread use, is inexpensive, and has few and mild side effects. Diabetes was reduced by 62% in the DREAM study, and this was [29] accompanied by a decreased risk of cardiovascular events (Fig. 12). Thus, compared with chemical drugs, Tianqi capsule was safe to use. A comparison of diabetes risk reduction between Tianqi and other large-scale clinical trials appears in Fig. 12.
Limitations
Our systematic review and meta-analysis had some limitations. Firstly, we had few studies to examine and limited statistical power to detect the differences across studies. Secondly, average follow-up for included trials was 1 year, which was similar to the SLIM study [30], but this is insufficient to assess long-term quality of life, diabetic microvascular complications, cardiovascular events, and all-cause mortality. Therefore, the long-term study of the effectiveness of Tianqi capsule for prevention of T2DM is necessary. Thirdly, the adverse events were not adequately documented. Fourthly, outcome measures should be focused on the TCM featured symptoms. Finally, study protocols were not published, and attrition bias could influence the results and lead to under- or overestimation. Thus, the results should be interpreted with caution.
Implications for Practice
As a result of the small number of included trials, a clinical recommendation cannot be warranted. Tianqi capsule appeared to be well tolerated in all included trials, but all trials were performed only on Chinese subjects, and no trial tested the intervention on other ethnic groups. Thus, the results may have limitations for generalizing to subjects outside of China. In TCM, IGT may fall under the patterns of “spleen pyretic abundance”. The main pathogenesis lies in spleen and stomach congestion, damp-heat accumulation in the spleen, and Qi stagnation due to liver depression leading to spleen qi deficiency in the body. If prolonged qi deficiency impairs yin, dual deficiency of qi and yin will occur [31]. Thus, different kinds of TCPMs may be chosen on the basis of the pattern differentiation in clinical practice.
Conclusions
The available evidence suggests that Tianqi capsule could be considered as a potential prevention of diabetes. The number and quality of available studies are insufficient. Larger, better-designed, and high-quality RCTs are required to provide stronger evidence in future studies.
Change history
03 November 2017
Incorrect author affiliation and the typo in acknowledgement section were found in the original publication. The correct author affiliations and acknowledgments are given here.
References
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care. 2015;38(Suppl. 1):S8–16.
Xu Y, Wang LM, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59.
Yang WY, Lu JM, Weng JP, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–101.
Yoon U, Kwok LL, Magkidis A. Efficacy of lifestyle interventions in reducing diabetes incidence in patients with impaired glucose tolerance: a systematic review of randomized controlled trials. Metabolism. 2013;62:303–14.
Phillips LS, Ratner RE, Buse JB, et al. We can change the natural history of type 2 diabetes. Diabetes Care. 2014;37:2668–76.
Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–44.
Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403.
American Diabetes Association. Standards of medical care in diabetes-2016: prevention or delay of type 2 diabetes. Diabetes Care. 2016;39(Suppl. 1):S36–8.
Merlotti C, Morabito A, Pontiroli AE. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes Metab. 2014;16:719–27.
Chinese Diabetes Society. Chinese guideline for type 2 diabetes in China (2013 edition). Chin J Diabetes. 2014;22:2–42.
Zhou QW, Zong RY, Xie XL, et al. Experimental study of Tianqi capsule on lowering the blood glucose. Chin Tradit Herb Drugs. 1997;28:95–8.
Zhao Q, Guo B, Yang W. Tianqi capsule to treat type 2 diabetes: a trial of 300 cases. J Shandong Univ Tradit Chin Med. 2003;27:191–2.
Cai HQ, Ge HQ, Zhang XJ, et al. 60 cases of clinical observation on Tianqi capsule treating type 2 diabetes mellitus. J Jilin Univ (Med Ed). 2003;29:669–71.
Higgins JPT, Green S. Corchrane reviewers’ Handbook 5.2 [updated March 2013], ReviewManager (RevMan) [Computer program]. Version 5.2. 2013.
Furlan AD, Pennick V, Bombardier C, van Tulder M, Editorial Board, Cochrane Back Review Group. Updated method guidelines for systematic reviews in the cochrane back review group. Spine (Phila Pa 1976). 2009;34:1929–41.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6.
Lian F, Li G, Chen X, et al. Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: a double-blind, randomized, placebo-controlled, multicenter trial. J Clin Endocrinol Metab. 2014;99:648–55.
Sun XF, Qu KY, Huang HT, et al. Tianqi Jiang Tang capsule for prevention of type 2 diabetes mellitus:a randomized, double-blind, placebo-controlled study in Chinese individuals with impaired glucose tolerance. Chin J Diabetes. 2011;19:433–6.
Wang YR, Tong XL, Xiao XH, et al. The therapeutic efficacy of Tianqi capsule intervention of patients with impaired glucose tolerance. Chin J Diabetes. 2011;19:525–8.
Hong SD, Hao JJ, Zhou ZJ, et al. Clinical research of treating impaired glucose tolerance by the intervention of traditional Chinese medicine combined with lifestyle modification. Chin Foreign Med Treat. 2013;21:115–6.
Wei Y. Clinical observation on Tianqi capsules in interventing impaired glucose tolerance. Dissertation, Zhejiang University of Traditional Chinese Medicine. 2009.
Wang Q. Clinical trial of integrated TCM therapy on 103 cases of IGT patients in Beijing. Dissertation, Beijing University of Traditional Chinese Medicine. 2010.
Grant SJ, Bensoussan A, Chang D, et al. Chinese herbal medicines for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev. 2009;4:CD006690.
Zhang SX, Sun H, Sun WJ, et al. Proteomic study of serum proteins in a type 2 diabetes mellitus rat model by Chinese traditional medicine Tianqi Jiangtang capsule administration. J Pharm Biomed Anal. 2010;53:1011–4.
Zhong HF, Liang QL, Luo GA, et al. Simultaneous LC-UV-MS-MS analysis of nine pivotal metabolites in human serum: application to studies of impaired glucose tolerance. Chromatographia. 2011;73:149–55.
Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289–97.
Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomized trial. Lancet. 2002;359:2072–7.
Gerstein HC, Yusuf S, Bosch J, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet. 2006;368:1096–105.
Corpeleijn E, Feskens EJ, Jansen EH, et al. Lifestyle intervention and adipokine levels in subjects at high risk for type 2 diabetes: the Study on Lifestyle intervention and Impaired glucose tolerance Maastricht (SLIM). Diabetes Care. 2007;30:3125–7.
China Association of Traditional Chinese Medicine. Guideline for TCM diabetes prevention and treatment. Beijing: Traditional Chinese Medicine Press of China; 2007.
Acknowledgements
This study is supported by a grant from the Major Program of National Natural Science Foundation of China (Grant no. 81430097). Article processing charges were paid by the Authors.
Bing Pang and Ting Zhang wrote the manuscriipt and are co-first authors; Yu-jiao Zheng and Li-sha He did the date collection and accessed the literature quality; Ying Zhang and Bing Pang performed the statistical analysis; Xiao-lin Tong, Feng-mei Lian and Jing Liu participated in the design and coordination of the study. Xiao-lin Tong and Feng-mei Lian are both corresponding authors. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosures
Bing Pang, Ying Zhang, Jing Liu, Li-sha He, Yu-jiao Zheng, Feng-mei Lian, and Xiao-lin Tong have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/E1BCF06010AF464D.
A correction to this article is available online at https://doi.org/10.1007/s13300-017-0331-y.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pang, B., Zhang, Y., Liu, J. et al. Prevention of Type 2 Diabetes with the Chinese Herbal Medicine Tianqi Capsule: A Systematic Review and Meta-Analysis. Diabetes Ther 8, 1227–1242 (2017). https://doi.org/10.1007/s13300-017-0316-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-017-0316-x