Abstract
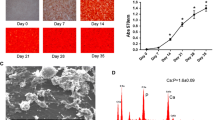
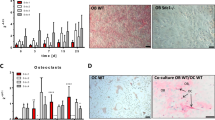
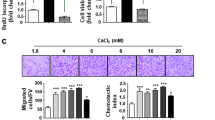
This study evaluated the temporal expression of osteopontin (OPN) in co-cultures of human osteoblastic cells (SAOS-2) and oral squamous cell carcinoma (OSCC)-derived cells (SCC9) and examined the effects of osteoblast-derived OPN on the neoplastic cell phenotype. Additionally, the effects of these co-cultures on subsequent osteoclastic activity were explored. SCC9 cells were plated on Transwell® membranes that were either coated or not coated with Matrigel and were then co-cultured with SAOS-2 cells during the peak of OPN expression. SCC9 cells exposed to OPN-silenced SAOS-2 cultures and SCC9 cells cultured alone served as controls. SCC9 cells were quantitatively evaluated for cell adhesion, proliferation, migration, and invasion into Matrigel. The impact of co-culturing SAOS-2 and SCC9 cells on the resorptive capacity of U-937-derived osteoclastic cells was also investigated. Furthermore, a reciprocal induction of SAOS-2 and SCC9 cells in terms of OPN expression over the co-culture interval was identified. SAOS-2-secreted OPN altered the SCC9 cell phenotype, leading to enhanced cell adhesion and proliferation and higher Matrigel invasion. This invasion was also enhanced, albeit to a lesser degree, by co-culture with OPN-silenced SAOS-2 cells. Cell migration was not affected. Co-culture with SAOS-2 cells—mainly during the period of peak OPN expression—promoted over-expression of IL-6 and IL-8 by SCC9 cells and enhanced the resorptive capacity of osteoclastic cells. Taken together, these results suggest that osteoblast-derived OPN affects the interactions among OSCC-derived epithelial cells, osteoblasts, and osteoclasts, which could contribute to the process of bone destruction during bone invasion by OSCC.





Similar content being viewed by others
References
Saman DM. A review of the epidemiology of oral and pharyngeal carcinoma: update. Head Neck Oncol. 2012;4:1.
Simard EP, Torre LA, Jemal A. International trends in head and neck cancer incidence rates: differences by country, sex and anatomic site. Oral Oncol. 2014;50:387–403.
Lichtenstein AV. Cancer: evolutionary, genetic and epigenetic aspects. Clin Epigenetics. 2010;1:85–100.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801.
Kellermann MG, Sobral LM, da Silva SD, Zecchin KG, Graner E, Lopes MA, et al. Mutual paracrine effects of oral squamous cell carcinoma cells and normal oral fibroblasts: induction of fibroblast to myofibroblast transdifferentiation and modulation of tumor cell proliferation. Oral Oncol. 2008;44:509–17.
Sobral LM, Zecchin KG, Nascimento de Aquino S, Lopes MA, Graner E, et al. Isolation and characterization of myofibroblast cell lines from oral squamous cell carcinoma. Oncol Rep. 2011;25:1013–20.
Sobral LM, Bufalino A, Lopes MA, Graner E, Salo T, Coletta RD. Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol. 2011;47:840–6.
Shevde LA, Samant RS, Paik JC, Metge BJ, Chambers AF, Casey G, et al. Osteopontin knockdown suppresses tumorigenicity of human metastatic breast carcinoma, MDA-MB-435. Clin Exp Metastasis. 2006;23:123–33.
Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–93.
Franzén A, Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985;232:715–24.
Weber GF, Cantor H. The immunology of Eta-1/osteopontin. Cytokine Growth Factor Rev. 1996;7:241–8.
Brown LF, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi CA, Manseau EJ, et al. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992;3:1169–80.
Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475–82.
Bautista DS, Denstedt J, Chambers AF, Harris JF. Low-molecular-weight variants of osteopontin generated by serine proteinases in urine of patients with kidney stones. J Cell Biochem. 1996;61:402–9.
Chen RX, Xia YH, Xue TC, Ye SL. Osteopontin promotes hepatocellular carcinoma invasion by up-regulating MMP-2 and uPA expression. Mol Biol Rep. 2011;38:3671–7.
Lu JG, Li Y, Li L, Kan X. Overexpression of osteopontin and integrin αv in laryngeal and hypopharyngeal carcinomas associated with differentiation and metastasis. J Cancer Res Clin Oncol. 2011;137:1613–8.
Wang ZM, Cui YH, Li W, Chen SY, Liu TS. Lentiviral-mediated siRNA targeted against osteopontin suppresses the growth and metastasis of gastric cancer cells. Oncol Rep. 2011;25:997–1003.
Zhang R, Pan X, Huang Z, Weber GF, Zhang G. Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS One. 2011;6:e23831.
Matsuzaki H, Shima K, Muramatsu T, Ro Y, Hashimoto S, Shibahara T, et al. Osteopontin as biomarker in early invasion by squamous cell carcinoma in tongue. J Oral Pathol Med. 2007;36:30–4.
Routray S, Kheur SM, Kheur M. Osteopontin: a marker for invasive oral squamous cell carcinoma but not for potentially malignant epithelial dysplasias. Ann Diagn Pathol. 2013;17:421–4.
Weber GF, Lett GS, Haubein NC. Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer. 2010;103:861–9.
Avirović M, Matušan-Ilijaš K, Damante G, Fabrro D, Cerović R, Juretić M, et al. Osteopontin expression is an independent factor for poor survival in oral squamous cell carcinoma: a computer-assisted analysis on TMA sections. J Oral Pathol Med. 2013;42:620–6.
Ingale Y, Routray S, Kheur SM, Kheur M, Mohanty N. Evaluating the efficacy of osteopontin expression as a prognostic marker in oral squamous cell carcinoma in the Indian subpopulation. J Oral Maxillofac Pathol. 2014;18(Suppl 1):S11–5.
Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303.
Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–84.
Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int. 2013;93:348–54.
Semba I, Matsuuchi H, Miura Y. Histomorphometric analysis of osteoclastic resorption in bone directly invaded by gingival squamous cell carcinoma. J Oral Pathol Med. 1996;25:429–35.
Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19:18–54.
Chellaiah MA, Kizer N, Biswas R, Alvarez U, Strauss-Schoenberger J, Rifas L, et al. Osteopontin deficiency produces osteoclast dysfunction due to reduced CD44 surface expression. Mol Biol Cell. 2003;14:173–89.
Li M, Amizuka N, Takeuchi K, Freitas PH, Kawano Y, Hoshino M, et al. Histochemical evidence of osteoclastic degradation of extracellular matrix in osteolytic metastasis originating from human lung small carcinoma (SBC-5) cells. Microsc Res Tech. 2006;69:73–83.
Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014;89:330–41.
Jimi E, Furuta H, Matsuo K, Tominaga K, Takahashi T, Nakanishi O. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis. 2011;17:462–8.
Amoui M, Suhr SM, Baylink DJ, Lau KH. An osteoclastic protein-tyrosine phosphatase may play a role in differentiation and activity of human monocytic U-937 cell-derived, osteoclast-like cells. Am J Phys Cell Phys. 2004;287:C874–84.
Schwartz Fo HO, Novaes Jr AB, de Castro LM, Rosa AL, de Oliveira PT. In vitro osteogenesis on a microstructured titanium surface with additional submicron-scale topography. Clin Oral Implants Res. 2007;18:333–44.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8.
de Oliva MA, Maximiano WM, de Castro LM, da Silva Jr PE, Fernandes RR, Ciancaglini P, et al. Treatment with a growth factor-protein mixture inhibits formation of mineralized nodules in osteogenic cell cultures grown on titanium. J Histochem Cytochem. 2009;57:265–76.
Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–18.
Terpos E, Kiagia M, Karapanagiotou EM, Charpidou A, Dilana KD, Nasothimiou E, et al. The clinical significance of serum markers of bone turnover in NSCLC patients: surveillance, management and prognostic implications. Anticancer Res. 2009;29:1651–7.
Weber GF, Lett GS, Haubein NC. Categorical meta-analysis of osteopontin as a clinical cancer marker. Oncol Rep. 2011;25:433–41.
Mardani M, Andisheh-Tadbir A, Khademi B, Fattahi MJ, Shafiee S, Asad-Zadeh M. Serum levels of osteopontin as a prognostic factor in patients with oral squamous cell carcinoma. Tumour Biol. 2014;35:3827–9.
Buijs JT, van der Pluijm G. Osteotropic cancers: from primary tumor to bone. Cancer Lett. 2009;273:177–93.
Samuvel DJ, Sundararaj KP, Li Y, Lopes-Virella MF, Huang Y. Adipocyte-mononuclear cell interaction, toll-like receptor 4 activation, and high glucose synergistically up-regulate osteopontin expression via an interleukin 6-mediated mechanism. J Biol Chem. 2010;285:3916–27.
Fried D, Mullins B, Weissler M, Shores C, Zanation A, Hackman T, et al. Prognostic significance of bone invasion for oral cavity squamous cell carcinoma considered T1/T2 by American joint committee on cancer size criteria. Head Neck. 2014;36:776–81.
Miki Y, Ono K, Hata S, Suzuki T, Kumamoto H, Sasano H. The advantages of co-culture over mono cell culture in simulating in vivo environment. J Steroid Biochem Mol Biol. 2012;131:68–75.
Gao C, Guo H, Downey L, Marroquin C, Wei J, Kuo PC. Osteopontin-dependent CD44v6 expression and cell adhesion in HepG2 cells. Carcinogenesis. 2003;24:1871–8.
Lyons AJ, Jones J. Cell adhesion molecules, the extracellular matrix and oral squamous carcinoma. Int J Oral Maxillofac Surg. 2007;36:671–9.
Harada T, Shinohara M, Nakamura S, Oka M. An immunohistochemical study of the extracellular matrix in oral squamous cell carcinoma and its association with invasive and metastatic potential. Virchows Arch. 1994;424:257–66.
Clëzardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr Cancer Drug Targets. 2009;9:801–6.
Schneider JG, Amend SR, Weilbaecher KN. Integrins and bone metastasis: integrating tumor cell and stromal cell interactions. Bone. 2011;48:54–65.
Angelucci A, Festuccia C, Gravina GL, Muzi P, Bonghi L, Vicentini C, et al. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate. 2004;59:157–66.
Nam TJ, Busby Jr WH, Rees C, Clemmons DR. Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth. Endocrinology. 2000;141:1100–6.
Duan C, Xu Q. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol. 2005;142:44–52.
Zhi X, Lamperska K, Golusinski P, Schork NJ, Luczewski L, Golusinski W, et al. Expression levels of insulin-like growth factors 1 and 2 in head and neck squamous cell carcinoma. Growth Hormon IGF Res. 2014;24:137–41.
Martinez C, Churchman M, Freeman T, Ilyas M. Osteopontin provides early proliferative drive and may be dependent upon aberrant c-myc signalling in murine intestinal tumours. Exp Mol Pathol. 2010;88:272–7.
Fukuda M, Hamao A, Tanaka A, Kitada M, Suzuki S, Kusama K, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/APO2L) and its receptors expression in human squamous cell carcinoma of the oral cavity. Oncol Rep. 2003;10:1113–9.
Kassouf N, Thornhill MH. Oral cancer cell lines can use multiple ligands, including Fas-L, TRAIL and TNF-alpha, to induce apoptosis in Jurkat T cells: possible mechanisms for immune escape by head and neck cancers. Oral Oncol. 2008;44:672–82.
Polat B, Wohlleben G, Katzer A, Djuzenova CS, Technau A, Flentje M. Influence of osteopontin silencing on survival and migration of lung cancer cells. Strahlenther Onkol. 2013;189:62–7.
Zhang H, Guo M, Chen JH, Wang Z, Du XF, Liu PX, et al. Osteopontin knockdown inhibits αv,β3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol Biochem. 2014;33:991–1002.
Agnihotri R, Crawford HC, Haro H, Matrisian LM, Havrda MC, Liaw L. Osteopontin, a novel substrate for matrix metalloproteinase-3 (stromelysin-1) and matrix metalloproteinase-7 (matrilysin). J Biol Chem. 2001;276:28261–7.
Gao YA, Agnihotri R, Vary CP, Liaw L. Expression and characterization of recombinant osteopontin peptides representing matrix metalloproteinase proteolytic fragments. Matrix Biol. 2004;23:457–66.
Chuang HC, Su CY, Huang HY, Huang CC, Chien CY, Du YY, et al. Active matrix metalloproteinase-7 is associated with invasion in buccal squamous cell carcinoma. Mod Pathol. 2008;21:1444–50.
Li TJ, Cui J. COX-2, MMP-7 expression in oral lichen planus and oral squamous cell carcinoma. Asian Pac J Trop Med. 2013;6:640–3.
Mäkinen LK, Häyry V, Hagström J, Sorsa T, Passador-Santos F, Keski-Säntti H, et al. Matrix metalloproteinase-7 and matrix metalloproteinase-25 in oral tongue squamous cell carcinoma. Head Neck. 2014;36:1783–8.
Mashhadiabbas F, Mahjour F, Mahjour SB, Fereidooni F, Hosseini FS. The immunohistochemical characterization of MMP-2, MMP-10, TIMP-1, TIMP-2, and podoplanin in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:240–50.
Gao ZB, Duan YQ, Zhang L, Chen DW, Ding PT. Expression of matrix metalloproteinase 2 and its tissue inhibitor in oral squamous cell carcinoma. Int J Mol Med. 2005;16:599–603.
Singh RD, Haridas N, Patel JB, Shah FD, Shukla SN, Shah PM, et al. Matrix metalloproteinases and their inhibitors: correlation with invasion and metastasis in oral cancer. Indian J Clin Biochem. 2010;25:250–9.
Singh RD, Nilayangode H, Patel JB, Shah FD, Shukla SN, Shah PM, et al. Combined evaluation of matrix metalloproteinases and their inhibitors has better clinical utility in oral cancer. Int J Biol Markers. 2011;26:27–36.
Pérez-Sayáns García M, Suárez-Peñaranda JM, Gayoso-Diz P, Barros-Angueira F, Gándara-Rey JM, García-García A. Tissue inhibitor of metalloproteinases in oral squamous cell carcinomas—a therapeutic target? Cancer Lett. 2012;323:11–9.
Bodnar M, Szylberg Ł, Kazmierczak W, Marszalek A. Tumor progression driven by pathways activating matrix metalloproteinases and their inhibitors. J Oral Pathol Med. 2015;44:437–43.
Li M, Wang Z, Xing Y, Yu J, Tian L, Zhang D, et al. A multicenter study on expressions of vascular endothelial growth factor, matrix metallopeptidase-9 and tissue inhibitor of metalloproteinase-2 in oral and maxillofacial squamous cell carcinoma. Iran Red Crescent Med J. 2014;16:e13185.
Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 1803;2010:55–71.
Erdem NF, Carlson ER, Gerard DA, Ichiki AT. Characterization of 3 oral squamous cell carcinoma cell lines with different invasion and/or metastatic potentials. J Oral Maxillofac Surg. 2007;65:1725–33.
Nakashima T, Yasumatsu R, Masuda M, Clayman GL, Komune S. Prognostic value of cathepsin L and its inhibitor headpin in oral squamous cell carcinoma. J Laryngol Otol. 2012;126:1134–7.
Bitu CC, Kauppila JH, Bufalino A, Nurmenniemi S, Teppo S, Keinänen M, et al. Cathepsin K is present in invasive oral tongue squamous cell carcinoma in vivo and in vitro. PLoS One. 2013;8:e70925.
Duong LT, Wesolowski GA, Leung P, Oballa R, Pickarski M. Efficacy of a cathepsin k inhibitor in a preclinical model for prevention and treatment of breast cancer bone metastasis. Mol Cancer Ther. 2014;13:2898–909.
Deyama Y, Tei K, Yoshimura Y, Izumiyama Y, Takeyama S, Hatta M, et al. Oral squamous cell carcinomas stimulate osteoclast differentiation. Oncol Rep. 2008;20:663–8.
Kayamori K, Sakamoto K, Nakashima T, Takayanagi H, Morita K, Omura K, et al. Roles of interleukin-6 and parathyroid hormone-related peptide in osteoclast formation associated with oral cancers: significance of interleukin-6 synthesized by stromal cells in response to cancer cells. Am J Pathol. 2010;176:968–80.
Hwang YS, Lee SK, Park KK, Chung WY. Secretion of IL-6 and IL-8 from lysophosphatidic acid-stimulated oral squamous cell carcinoma promotes osteoclastogenesis and bone resorption. Oral Oncol. 2012;48:40–8.
Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37.
Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone. 2003;32:1–7.
Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60.
Gilbert M, Giachelli CM, Stayton PS. Biomimetic peptides that engage specific integrin-dependent signaling pathways and bind to calcium phosphate surfaces. J Biomed Mater Res A. 2003;67:69–77.
Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM, Stayton PS. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J Biol Chem. 2000;275:16213–8.
Pietak AM, Sayer M. Functional atomic force microscopy investigation of osteopontin affinity for silicon stabilized tricalcium phosphate bioceramic surfaces. Biomaterials. 2006;27:3–14.
Acknowledgments
The authors would like to thank the State of Sao Paulo Research Foundation (FAPESP, grants #2012/07531-9, #2012/20863-0, and #2012/08605-6) and the National Council of Scientific and Technological Development (CNPq, grant #308200/2012-8) for financial support. Roger Rodrigo Fernandes and Fabíola Singaretti de Oliveira are acknowledged for technical assistance. The mouse monoclonal anti-rat osteopontin (MPIIIB10-1) antibody, developed by Michael Solursh and Ahnders Franzen, was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
The State of Sao Paulo Research Foundation (FAPESP, Brazil), grants #2012/07531-9, #2012/20863-0, and #2012/08605-6, and the National Council of Scientific and Technological Development (CNPq, Brazil), grant #308200/2012-8.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Teixeira, L.N., de Castro Raucci, L.M.S., Alonso, G.C. et al. Osteopontin expression in co-cultures of human squamous cell carcinoma-derived cells and osteoblastic cells and its effects on the neoplastic cell phenotype and osteoclastic activation. Tumor Biol. 37, 12371–12385 (2016). https://doi.org/10.1007/s13277-016-5104-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5104-0