Abstract
Although SMAD3 signaling has been suggested to play a role in the metastasis of various cancers, its possible involvement as well as the underlying mechanism in the pathogenesis in prostate cancer remains unclear. Here, we found that the MMP9 level, an indicator of the invasiveness of cancer cells, negatively correlates with the activity of phosphorylated SMAD3 levels in the prostate cancer patients. Moreover, the phosphorylated SMAD3 also appeared to regulate the MMP9 level in a prostate cancer cell line, PC3. Augmented phosphorylated SMAD3 inhibited MMP9 and invasiveness of PC3 cells, while inhibition of phosphorylated SMAD3 activated MMP9 and promoted PC3 cell invasiveness. Furthermore, forced MMP9 inhibition abolished the effect of phosphorylated SMAD3 on the invasiveness of PC3 cells, while forced MMP9 activation abolished the effect of phosphorylated SMAD3 on the invasiveness of PC3 cells. Taken together, our data suggest the possibility of the existence of a unique signaling cascade in which SMAD3 signaling regulates MMP9 during cancer metastasis.
Similar content being viewed by others
Introduction
Prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Most prostate cancers are slow growing. However, there are cases of aggressive prostate cancers. In such situation, the cancer cells may metastasize from the prostate to other parts of the body, particularly the bones and lymph nodes [1–3].
Transforming growth factor beta (TGF-beta) signaling pathways are essential for many biological events [4–9]. There are TGF-beta types I and II receptors. When a TGF-beta ligand binds to a type II receptor, it catalyzes the phosphorylation of a type I receptor, and subsequently triggers phosphorylation of intracellular proteins called receptor-regulated SMADs (R-SMADs), which form heteromeric complexes with the common-mediator SMAD (Co-SMAD), SMAD4. The activated SMAD complexes translocate to the nucleus, where they regulate the transcription of target genes. R-SMADs 2 and 3 typically mediate TGF-beta signaling [4–9]. Although TGF-beta signaling pathway has been shown to play a critical and somehow complicated role during tumor pathogenesis, its exact role in the metastasis of prostate cancer cells has not been adequately studied.
In the current study, we found that the MMP9 level, an indicator of the invasiveness of cancer cells, negatively correlates with the activity of phosphorylated SMAD3 levels in the prostate cancer patients. Moreover, the phosphorylated SMAD3 also appeared to regulate the MMP9 level in a prostate cancer cell line, PC3. Augmented phosphorylated SMAD3 inhibited MMP9 and invasiveness of PC3 cells, while inhibition of phosphorylated SMAD3 activated MMP9 and promoted PC3 cell invasiveness. Furthermore, forced MMP9 inhibition abolished the effect of phosphorylated SMAD3 on the invasiveness of PC3 cells, while forced MMP9 activation abolished the effect of phosphorylated SMAD3 on the invasiveness of PC3 cells. Taken together, our data suggest the possibility of the existence of a unique signaling cascade in which pSMAD3 signaling regulates MMP9 to promote prostate cancer metastasis. Additional experiments are needed to further confirm it.
Materials and methods
Patient tissue specimens
A total of 37 resected specimens from prostate cancer patients were collected for this study. All specimens had been histologically and clinically diagnosed at the Department of Endocrinology and Surgery of The First Affiliated Hospital, ZheJiang University, from 2007 to 2013. For the use of these clinical materials for research purposes, prior patient’s consents and approval from the Institutional Research Ethics Committee were obtained.
Preparation of cell lines
Human prostate cancer cell line PC3 has been described before [10] and was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (PAA, Austria). All overexpressing or ShRNA plasmids (2 μg) were produced by Genema (Shanghai, China). To adapt the expression levels of phosphorylated SMAD3 or MMP9 in PC3 cells, we infected the cells with a recombinant lentivirus expressing the transgene or ShRNA under the control of CMV promoter at MOI 100.
Transwell matrix penetration assay
Cells (5 × 105) were plated into the top side of polycarbonate transwell filter coated with Matrigel in the upper chamber of the BioCoatTM Invasion Chambers (BD, Bedford, MA, USA) and incubated at 37 °C for 22 h. The cells inside the upper chamber with cotton swabs were then removed. Migratory and invasive cells on the lower membrane surface were fixed, stained with hematoxylin, and counted for 10 random ×100 fields per well. Cell counts are expressed as the mean number of cells per field of view.
ELISA assay
The concentration of MMP9 in the conditioned medium from cultured cells was determined by a MMP9 ELISA Kit (Calbiochem/Oncogene, Cambridge, MA, USA). ELISAs were performed according to the instructions of the manufacturer. Briefly, the collected condition medium was added to a well coated with MMP9 polyclonal antibody, and then immunosorbented by biotinylated monoclonal anti-human MMP9 antibody at room temperature for 2 h. The color development catalyzed by horseradish peroxidase was terminated with 2.5 mol/l sulfuric acid, and the absorption was measured at 450 nm. The protein concentration was determined by comparing the relative absorbance of the samples with the standards.
RNA extraction, reverse transcription, and RT-qPCR
Total RNA was extracted from the cultured cells using RNAeasy kit (Invitrogen), according to the manufacturer’s instruction. For messenger RNA (mRNA) analysis, complementary DNA (cDNA) was randomly primed from 2 μg of total RNA using the Omniscript Reverse Transcription Kit (Qiagen). Real-time quantitative PCR (RT-qPCR) was subsequently performed in triplicate with a 1:4 dilution of cDNA using the QuantiTect SYBR Green PCR system (Qiagen) on a RotorGene 6000 series PCR machine (Corbett Research). Data were collected and analyzed using the RotorGene software accompanying the PCR machine. Relative expression levels were determined using the comparative quantification feature of the RotorGene software. All mRNA quantification data were normalized to α-Tubulin. PCR primers were generated by Genema.
Western blot
Protein was extracted from the cultured cells by RIPA buffer (Sigma, St. Louis, USA) for Western blot. An equal amount of proteins was loaded in the gel. Primary antibodies for Western blot are rabbit SMAD3, phosphorylated-SMAD3 (pSMAD3), MMP9, and α-Tubulin (Cell Signaling, LA, USA). Secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs, LA, USA). Images shown in the figure were representative from four repeats in one group.
Statistical analysis
Each experiment condition contains four repeats. All values are depicted as mean ± standard deviation and are considered significant if p < 0.05. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction. Bivariate correlations between pSMAD3 and MMP9 were calculated by Spearman’s rank correlation coefficients.
Results
pSMAD3 correlates with MMP9 level and cancer invasiveness in prostate cancer patients
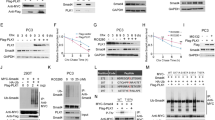
Since MMPs are closely related to tumor invasiveness, we thus examined the different levels of MMPs in the resected prostate cancer tissue from the patients by Western blot. Among all MMPs that have been examined, we found that MMP9 had a significantly higher level in the prostate cancer tissue. Therefore, we focused on MMP9 in our study. In order to evaluate the possible causal link between the levels of TGF-beta signaling and cancer metastasis, we examined MMP9 and phosphorylated SMAD3 (pSMAD3) levels in the resected prostate cancer tissue from 37 prostate cancer patients by Western blot. Six specimens were randomly selected and shown as representative images (Fig. 1a). We did not examine the phosphorylated form of another potential component of TGF-beta signaling, SMAD2, since we and others did not find its significant expression in the prostate cancer cells [11, 12]. A strong negative correlation was detected between the levels of MMP9 and pSMAD3 (Fig. 1b, ɤ 2 = 0.64; p < 0.0001), suggesting that the MMP9 levels may be affected by TGF-beta signaling in prostate cancer cells.
Inhibition of pSMAD3 signaling correlates with an increase in MMP9 level and cancer invasiveness in prostate cancer patients. a Representative images of Western blot of MMP9, SMAD3, pSMAD3, and α-Tubulin on the resected cancer tissue from prostate cancer patients. b A strong correlation was detected (ɤ 2 = 0.64; p < 0.0001), suggesting that the MMP9 levels may be affected by pSMAD3 signaling in prostate cancer patients
Establishment of PC3 cells with adapted pSMAD3
In order to examine whether enhanced TGF-beta signaling may inhibit expression of MMP9 and consequently the prostate cancer cell invasiveness, we infected a human prostate cancer cell line, PC3, with a lentiviral vector carrying SMAD3 transgene under CMV promoter. Similarly, we also generated a lentivirus that expresses SMAD3 shRNA (ShSMAD3) to inhibit its expression. Control virus only expressed null insert. The stable clones that express null insert (control: PC3-CTL), or SMAD3 (PC3-SMAD3), or ShSMAD3 (PC3-ShSMAD3) were established (Fig. 2a).
pSMAD3 controls protein and mRNA levels of MMP9 in PC3 cells. a Establishment of PC3 cells with adapted pSMAD3 and adapted MMP levels. b Western blot of MMP9, SMAD3, pSMAD3, and α-Tubulin on the modulated PC3 cells. c RT-qPCR of MMP9 (normalized to α-Tubulin, then compared with levels in PC3-CTL) on the modulated PC3 cells. *p < 0.05
We found that overexpression of SMAD3 in PC3 cells (PC3-SMAD3) significantly increased the phosphorylated form of SMAD3, which represented an enhance TGF-beta signaling (Fig. 2b). Similarly, SMAD3 inhibition in PC3 cells (PC3-ShSMAD3) significantly decreased the phosphorylated form of SMAD3, which represented an abolished TGF-beta signaling (Fig. 2b).
pSMAD3 inhibited MMP9 and invasiveness of PC3 cells
The expression of MMP9 in PC3-SMAD3 was evaluated and shown significant decrease by Western blot (Fig. 2b) and by RT-qPCR (Fig. 2c). Moreover, a significant decrease in MMP9 was detected in the conditioned medium by ELISA (Fig. 3a), suggesting that enhanced pSMAD3 inhibited MMP9 production and secretion. We also detected a significantly decreased invasiveness of the PC3-SMAD3 cells in a Transwell matrix penetration assay, suggesting that the decreased MMP9 levels to enhanced pSMAD3 resulted in a decrease in the invasiveness of the PC3 cells (Fig. 3b).
Inhibition of pSMAD3 activated MMP9 and promoted PC3 cell invasiveness
The expression of MMP9 was also evaluated in PC3-ShSMAD3, showing a significant increase by Western blot (Fig. 2b) and by RT-qPCR (Fig. 2c). Moreover, a significant increase in MMP9 was detected in the conditioned medium by ELISA (Fig. 3a), suggesting that inhibition of pSMAD3 activated MMP9 production and secretion. When the invasiveness of the PC3-ShSMAD3 cells was examined in a Transwell matrix penetration assay, we found that these cells significantly increased their invasiveness. These data suggest that the increased MMP9 levels by the abolished pSMAD3 signaling resulted in an increase in the invasiveness of the PC3 cells (Fig. 3b).
MMP9 inhibition abolished the effect of inhibition of pSMAD3 on the invasiveness of PC3 cells
In order to find out whether there is a casual link between pSMAD3 and MMP9 activation, we infected PC3 with a lentiviral vector carrying both ShSMAD3 and MMP9 shRNA (ShMMP9) under CMV promoter. Similarly, we also generated a lentivirus that expresses SMAD3 and MMP9 transgene. Control virus only expressed null insert. The stable clones that express ShSMAD3 and ShMMP9 (PC3-ShSMAD3-ShMMP9) or SMAD3 and MMP (PC3-SMAD3-MMP9) were established, to compare with the PC3-CTL, PC3-SMAD3, and PC3-ShSMAD3 (Fig. 2a).
We found that inhibition of MMP9 by ShMMP9 in PC3 cells (PC3-ShSMAD3-ShMMP9) significantly inhibited MMP9 levels without affecting the pSMAD3. On the other hand, increased MMP9 by MMP9 transgene in PC3 cells (PC3-SMAD3-MMP9) significantly increased MMP9 levels without affecting the pSMAD3 (Figs. 2b, c and 3a). These data suggest that MMP9 may be downstream of pSMAD3 signaling, since its expression does not seem to affect SMAD3 levels. Moreover, inhibition of MMP9 by ShMMP9 in PC3 cells (PC3-ShSMAD3-ShMMP9) significantly abrogated the increase in the PC3 cell invasiveness in the Transwell matrix penetration assay (Fig. 3b).
MMP9 activation abolished the effect of enhanced pSMAD3 on the invasiveness of PC3 cells
Similarly, the increase in MMP9 by MMP9 transgene in PC3 cells (PC3-SMAD3-MMP9) significantly abrogated the decrease in the PC3 cell invasiveness in the Transwell matrix penetration assay (Fig. 3b). Taken together, our data suggest the possibility of the existence of a unique signaling cascade in which pSMAD3 signaling regulates MMP9 to promote prostate cancer metastasis. Additional experiments are needed to further confirm it.
Discussion
The active involvement of TGF-beta signaling in the metastasis in various human tumors has been reported. However, the related studies in prostate cancer metastasis are very lacking. Here, we found that the MMP9 level, an indicator of the invasiveness of cancer cells, negatively correlates with the activity of TGF-beta signaling (pSMAD3 level) in the prostate cancer patients. This finding prompted us to define whether these is a causal link between MMP9 and pSMAD3. With a set of gain-of-function and loss-of-function experiments, we found that the pSMAD3 appeared to regulate the MMP levels in a prostate cancer cell line, PC3. Augmented pSMAD3 inhibited MMP9 and invasiveness of PC3 cells, while inhibition of pSMAD3 activated MMP9 and promoted PC3 cell invasiveness. Furthermore, MMP9 inhibition abolished the effect of inhibition of pSMAD3 signaling on the invasiveness of PC3 cells, while MMP9 activation abolished the effect of enhanced pSMAD3 signaling on the invasiveness of PC3 cells. Of note, we have indeed checked another prostatic cancer cell line DU-145 to exclude the possibility of cell line-dependent phenomenon detected in the current study. Taken together, our data suggest the possibility of the existence of a unique signaling cascade in which pSMAD3 signaling regulates MMP9 to promote prostate cancer metastasis. Our study thus bridges the pSMAD3 signaling pathway and MMP9 activity in the prostate cancer metastasis, suggesting that the interplay between pSMAD3 signaling and MMP9 may be a novel prostate cancer invasion model to be targeted for prostate cancer therapy.
References
Olsson AY, Cooper CS. The molecular basis of prostate cancer. Br J Hosp Med (Lond). 2005;66:612–6.
Powell IJ, Bollig-Fischer A. Minireview: The molecular and genomic basis for prostate cancer health disparities. Mol Endocrinol. 2013;27:879–91.
Mazaris E, Tsiotras A. Molecular pathways in prostate cancer. Nephro-Urol Mon. 2013;5:792–800.
Massague J. Tgfbeta in cancer. Cell. 2008;134:215–30.
Shi Y, Massague J. Mechanisms of tgf-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700.
Kretzschmar M, Massague J. Smads: mediators and regulators of tgf-beta signaling. Curr Opin Genet Dev. 1998;8:103–11.
Massague J, Chen YG. Controlling tgf-beta signaling. Genes Dev. 2000;14:627–44.
Massague J. Tgfbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, et al. M2 macrophages promote beta-cell proliferation by up-regulation of smad7. Proc Natl Acad Sci U S A. 2014;111:E1211–20.
Huang SQ, Liao QJ, Wang XW, Xin DQ, Chen SX, Wu QJ, et al. Rnai-mediated knockdown of pituitary tumor- transforming gene-1 (pttg1) suppresses the proliferation and invasive potential of pc3 human prostate cancer cells. Braz J Med Biol Res. 2012;45:995–1001.
Huang S, Liao Q, Li L, Xin D. Tumour Biol. 2014. doi:10.1007/s13277-014-1818-z.
Zhang J, Wang Y, Li D, Jing S. Notch and tgf-beta/smad3 pathways are involved in the interaction between cancer cells and cancer-associated fibroblasts in papillary thyroid carcinoma. Tumour Biol. 2014;35:379–85.
Acknowledgments
This work was financially supported by the Department of Health of Zhejiang Province (No. 201234615).
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Qing Xia and Chengjiang Li have equal contribution.
Rights and permissions
About this article
Cite this article
Xia, Q., Li, C., Bian, P. et al. Targeting SMAD3 for inhibiting prostate cancer metastasis. Tumor Biol. 35, 8537–8541 (2014). https://doi.org/10.1007/s13277-014-2368-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2368-0