Abstract
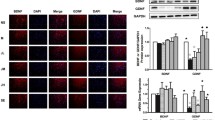
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by loss of dopaminergic neurons in the substantia nigra pars compacta. In this study, we investigated the effects of a novel herb formula, Hepad, on PD. Dose-dependent treatment with 1-methyl-4-phenylpyridinium (MPP+) decreased the viability of SH-SY5Y cells, and Hepad inhibited the toxic effect of MPP+. Hepad blocked the production of reactive oxygen species (ROS) induced by MPP+ in SH-SY5Y cells, and suppressed the activation of caspase 9 and caspase 3 due to MPP+. A rat model of PD was generated by 6-hydroxydopamine (6-OHDA) injection into the left medial forebrain bundle (MFB) of SD rats. In D-amphetamine sulfate-induced rotational behavioral tests, Hepad administration attenuated circling behavior relative to the 6-OHDA-treated disease group. In addition, Hepad treatment significantly increased the tyrosine hydroxylase (TH)-positive cells in the substantia nigra pars compacta (SNpc) that had decreased in response to 6-OHDA treatment (P<0.05). OX-6 expression, which indicates the presence of microglial cells, decreased significantly after treatment of Hepad in contrast to the 6-OHDA-treated disease group (P<0.05). These results indicate that Hepad may be a useful neuroprotective material for the treatment of neurodegenerative disorders such as PD.
Similar content being viewed by others
References
de Lau, L. M. and Breteler, M. M. Epidemiology of Par-kinson’s disease. Lancet Neurol 5:525–535 (2006).
Zhao, Y. et al. GDNF-transfected macrophages pro-duce potent neuroprotective effects in Parkinson’s dis-ease mouse model. PLoS One 9:e106867 (2014).
Owen, A. D., Schapira, A. H., Jenner, P. & Marsden, C. D. Oxidative stress and Parkinson’s disease. Ann N Y Acad Sci 15:217–223 (1996).
Kidd, P. M. Parkinson’s disease as multifactorial oxi-dative neurodegeneration: implications for integrative management. Altern Med Rev 5:502–529 (2000).
Jain, S., Wood, N. W. & Healy, D. G. Molecular genetic pathways in Parkinson’s disease: a review. Clin Sci (Lond) 109:355–364 (2005).
Nicotra, A. & Parvez, S. Apoptotic molecules and MPTP-induced cell death. Neurotoxicol Teratol 24:599–605 (2002).
Alberio, T., Lopiano, L. & Fasano, M. Cellular models to investigate biochemical pathways in Parkinson’s disease. FEBS J 279:1146–1155 (2012).
Blandini, F. & Armentero, M. T. Animal models of par-kinson’s disease. FEBS J 279:1156–1166 (2012).
Chotibut, T., Apple, D. M., Jefferis, R. & Salvatore, M. F. Dopamine transporter loss in 6-OHDA Parkinson’s model is unmet by parallel reduction in dopamine up-take. PLoS One 7:e52322 (2012).
Connolly, B. S. & Lang, A. E. Pharmacological treat-ment of Parkinson disease: a review. JAMA 311:1670–1683 (2014).
Shulman, L. M., Taback, R. L., Bean, J. & Weiner, W. J. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord 16:507–510 (2001).
Li, X. Z., Zhang, S. N., Liu, S. M. & Lu, F. Recent ad-vances in herbal medicines treating Parkinson’s disease. Fitoterapia 84:273–285 (2013).
Adams, J. D., Klaidman, L. K. & Leung, A. C. MPP+ and MPDP+ induced oxygen radical formation with mitochondrial enzymes. Free Radic Biol Med 15:181–186 (1993).
Lee, B. et al. Protective effects of methanol extract of Acori graminei rhizoma and Uncariae Ramulus et Un-cus on ischemia-induced neuronal death and cognitive impairments in the rat. Life Sci 74:435–450 (2003).
Kim, J. H. et al. Effects of methanol extract of Uncari-ae Ramulus et Uncus on ibotenic acid-induced amnesia in the rat. J Pharmacol Sci 96:314–323 (2004).
Hiratsuka, T. et al. Yokukansan inhibits neuronal death during ER stress by regulating the unfolded protein re-sponse. PLoS One 5:e13280 (2010).
Hirsch, E. C. & Hunot, S. Neuroinflammation in Par-kinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397 (2009).
Hirsch, E. C., Vyas, S. & Hunot, S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord 18 Suppl 1:S210-S212 (2012).
Kim, J., Park, C. S., Lim, Y. & Kim, H. S. Paeonia ja-ponica, Houttuynia cordata, and Aster scaber water extracts induce nitric oxide and cytokine production by lipopolysaccharide-activated macrophages. J Med Food 12:365–373 (2009).
Chen, G. et al. Er-Miao-San, a traditional herbal for-mula containing Rhizoma Atractylodis and Cortex Phel-lodendri inhibits inflammatory mediators in LPS-stim-ulated RAW264.7 macrophages through inhibition of NF-kB pathway and MAPKs activation. J Ethnophar-macol 154:711–718 (2014).
Jeong, J. W. et al. Ethanol extract of Poria cocos redu-ces the production of inflammatory mediators by sup-pressing the NF-kappaB signaling pathway in lipopoly-saccharide-stimulated RAW 264.7 macrophages. BMC Complement Altern Med 14:101 (2014).
Lu, Y. T., Kuan, Y. C., Chang, H. H. & Sheu, F. Molec-ular cloning of a Poria cocos protein that activates Th1 immune response and allays Th2 cytokine and IgE pro-duction in a murine atopic dermatitis model. J Agric Food Chem 62:2861–2871 (2014).
Dauer, W. & Przedborski, S. Parkinson’s disease: mech anisms and models. Neuron 39:889–909 (2003).
Kim, S. W., Ko, H. S., Dawson, V. L. & Dawson, T. M. Recent advances in our understanding of Parkinson’s disease. Drug Discovery Today: Disease Mechanisms 2: 427–433 (2005).
Zhang, Z. G. et al. Astragaloside IV prevents MPP+-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol Cell Biochem 364: 209–216 (2012).
Tseng, Y. T., Chang, F. R. & Lo, Y. C. The Chinese herbal formula Liuwei dihuang protects dopaminergic neurons against Parkinson’s toxin through enhancing antioxidative defense and preventing apoptotic death. Phytomedicine 21:724–733 (2014).
Doo, A. R. et al. Neuroprotective effects of an herbal medicine, Yi-Gan San on MPP+/MPTP-induced cyto-toxicity in vitro and in vivo. J Ethnopharmacol 131: 433–442 (2010).
Bae, N. et al. The neuroprotective effect of modified Yeoldahanso-tang via autophagy enhancement in mod-els of Parkinson’s disease. J Ethnopharmacol 134:313–322 (2011)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Baek, S.Y., Lee, N.R., Kim, D.H. et al. Protective effect of a novel herbmedicine, Hepad, on apoptosis of SH-SY5Y cells and a rat model of Parkinson’s disease. Mol. Cell. Toxicol. 11, 223–230 (2015). https://doi.org/10.1007/s13273-015-0021-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-015-0021-7