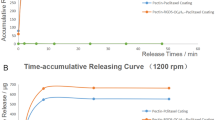
Abstract
Drug coated balloons (DCB) are becoming the standard-care treatment for peripheral arterial disease (PAD). DCB use excipients to transfer and retain anti-proliferative drugs, such as paclitaxel. Excipients thus play a vital role in the design and function of DCB, however methods to coat balloons with excipients and anti-proliferative drugs remain unknown. The goal of this study was to thus develop an approach to coat and evaluate DCB for various excipients. An air sprayer method was developed to deposit paclitaxel and various excipients onto non-coated commercially available angioplasty balloons. The coating of the angioplasty balloons was evaluated for drug deposition and coating efficiency using high performance liquid chromatography tandem mass spectrometry. Drug transfer and retention of the coated angioplasty balloons into arterial segments were evaluated ex vivo using harvested pig arteries in a pulsatile flow bioreactor. The air sprayer method successfully delivered varying excipients including bovine serum albumin (BSA), urea and iohexol. The air spray method was configured to coat four angioplasty balloons simultaneously with paclitaxel and iohexol with an average paclitaxel load of 4.0 ± 0.70 µg/mm2. The intra-day (within) and inter-day (between) coating precisions, defined as relative standard deviation (RSD), was 17.2 and 15.5%, respectively. Ex vivo deployment of iohexol-paclitaxel DCB yielded an arterial paclitaxel concentration of 123.4 ± 44.68 ng/mg (n = 3) at 1 h, 126.7 ± 25.27 ng/mg (n = 3) at 1 day, and 12.9 ± 12.88 ng/mg (n = 3) at 7 days. This work provides proof-of-concept of a quick, inexpensive approach to coat commercially available angioplasty balloons with paclitaxel and various excipients.






Similar content being viewed by others
References
Anderson, J. A., S. Lamichhane, T. Remund, P. Kelly, and G. Mani. Preparation, characterization, in vitro drug release, and cellular interactions of tailored paclitaxel releasing polyethylene oxide films for drug-coated balloons. Acta Biomater. 29:333–351, 2016.
Ansari, F., L. K. Pack, S. S. Brooks, and T. M. Morrison. Design considerations for studies of the biomechanical environment of the femoropopliteal arteries. J. Vasc. Surg. 58:804–813, 2013.
Atigh, M. K., E. A. Turner, U. Christians, and S. K. Yazdani. The use of an occlusion perfusion catheter to deliver paclitaxel to the arterial wall. Cardiovasc. Therap. 2017. https://doi.org/10.1111/1755-5922.12269.
Axel, D. I., W. Kunert, C. Goggelmann, M. Oberhoff, C. Herdeg, A. Kuttner, D. H. Wild, B. R. Brehm, R. Riessen, F. Koveker, and K. R. Karsch. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 96:636–645, 1997.
Blessing, E. The next generation of drug-coated balloons: the PANTHER study. Suppl. Endovasc. Today Eur. 3:12–14, 2015.
Bosiers, M., K. Deloose, J. Callaert, K. Keirse, J. Verbist, and P. Peeters. Drug-eluting stents below the knee. J. Cardiovasc. Surg. 52:231–234, 2011.
Buszman, P. P., A. Tellez, M. Afari, Y. Cheng, G. B. Conditt, J. C. McGregor, K. Milewski, M. Stenoien, G. L. Kaluza, and J. F. Granada. Stent healing response following delivery of paclitaxel via durable polymeric matrix versus iopromide-based balloon coating in the familial hypercholesterolaemic swine model of coronary injury. EuroIntervention. 9:510–516, 2013.
Chesher, D. Evaluating assay precision. Clin. Biochem. Rev. 29:S23–S26, 2008.
Cortese, B., A. Micheli, A. Picchi, A. Coppolaro, L. Bandinelli, S. Severi, and U. Limbruno. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomized clinical trial. Heart. 96:1291–1296, 2010.
Cremers, B., Y. Clever, S. Schaffner, U. Speck, M. Böhm, and B. Scheller. Treatment of coronary in-stent restenosis with a novel paclitaxel urea coated balloon. Minerva Cardioangiol. 58:583–588, 2010.
Fan, Y., L. Marlin, R. A. Sahatjian, and S. A. Schultz. Medical device with lubricious coating., 1996.
Fernandez-Parra, R., A. Laborda, C. Lahuerta, F. Lostale, J. Aramayona, I. de Blas, and M. A. de Gregorio. Pharmacokinetic study of paclitaxel concentration after drug-eluting balloon angioplasty in the iliac artery of healthy and atherosclerotic rabbit models. J. Vasc. Interv. Radiol. 26(1380–7):e1, 2015.
Fortier, A., V. Gullapalli, and R. A. Mirshams. Review of biomechanical studies of arteries and their effect on stent performance. IJC Heart Vessel. 4:12–18, 2014.
Gandhi, P. J., and Z. V. P. Murthy. Kinetic study of ultrasonic antisolvent crystallization of sirolimus. Cryst. Res. Technol. 45:321–327, 2010.
Gandhi, P. J., and Z. V. P. Murthy. Investigation of different drug deposition techniques on drug releasing properties of cardiovascular drug coated balloons. Ind. Eng. Chem. Res. 51:10800–10823, 2012.
Go, A. S., D. Mozaffarian, V. L. Roger, E. J. Benjamin, J. D. Berry, M. J. Blaha, S. Dai, E. S. Ford, C. S. Fox, S. Franco, H. J. Fullerton, C. Gillespie, S. M. Hailpern, J. A. Heit, V. J. Howard, M. D. Huffman, S. E. Judd, B. M. Kissela, S. J. Kittner, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, R. H. Mackey, D. J. Magid, G. M. Marcus, A. Marelli, D. B. Matchar, D. K. McGuire, E. R. Mohler, 3rd, C. S. Moy, M. E. Mussolino, R. W. Neumar, G. Nichol, D. K. Pandey, N. P. Paynter, M. J. Reeves, P. D. Sorlie, J. Stein, A. Towfighi, T. N. Turan, S. S. Virani, N. D. Wong, D. Woo, and M. B. Turner. American Heart Association Statistics C and Stroke Statistics S. Executive summary: heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 129:399–410, 2014.
Granada, J. F., M. Stenoien, P. P. Buszman, A. Tellez, D. Langanki, G. L. Kaluza, M. B. Leon, W. Gray, M. R. Jaff, and R. S. Schwartz. Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: impact on neointimal proliferation and healing. Open Heart. 1:e000117, 2014.
Hehrlein, C., G. Richardt, M. Wiemer, H. Schneider, C. Naber, E. Hoffmann, and U. Dietz. Description of Pantera Lux paclitaxel-releasing balloon and preliminary quantitative coronary angiography (QCA) results at six months in patients with coronary in-stent restenosis. EuroIntervention. 7(Suppl K):K119–K1124, 2011.
Hirsch, A. T., L. Hartman, R. J. Town, and B. A. Virnig. National health care costs of peripheral arterial disease in the Medicare population. Vasc. Med. 13:209–215, 2008.
Kaule, S., I. Minrath, F. Stein, U. Kragl, W. Schmidt, K. P. Schmitz, K. Sternberg, and S. Petersen. Correlating coating characteristics with the performance of drug-coated balloons: a comparative in vitro investigation of own established hydrogel- and ionic liquid-based coating matrices. PLoS ONE 10:e0116080, 2015.
Kelsch, B., B. Scheller, M. Biedermann, Y. P. Clever, S. Schaffner, D. Mahnkopf, U. Speck, and B. Cremers. Dose response to Paclitaxel-coated balloon catheters in the porcine coronary overstretch and stent implantation model. Invest. Radiol. 46:255–263, 2011.
Kothwala, D., A. Raval, A. Choubey, C. Engineer, and H. Kotadia. Paclitaxel drug delivery from cardoivascular stent. Trends Biomater. Artif. Organs. 19:88–92, 2006.
Kumar, G. N., U. K. Walle, K. N. Bhalla, and T. Walle. Binding of taxol to human plasma, albumin and alpha 1-acid glycoprotein. Res. Commun. Chem. Pathol. Pharmacol. 80:337–344, 1993.
Lamichhane, S., J. Anderson, T. Remund, P. Kelly, and G. Mani. Dextran sulfate as a drug delivery platform for drug-coated balloons: preparation, characterization, in vitro drug elution, and smooth muscle cell response. J. Biomed. Mater. Res. B Appl. Biomater. 104:1416–1430, 2016.
Liistro, F. Drug-eluting balloons are better for below-knee lesions than sirolimus-eluting stents. Cath. Lab. Digest. 21, 2013.
Maca, T., R. Ahmadi, K. Derfler, W. Hörl, R. Koppensteiner, E. Minar, B. Schneider, A. Stümpflen, and H. Ehringer. Elevated lipoprotein(a) and increased incidence of restenosis after femoropopliteal PTA: rationale for the higher risk of recurrence in females? Atherosclerosis. 127:27–34, 1996.
Margolis, J., J. McDonald, R. Heuser, P. Klinke, R. Waksman, R. Virmani, N. Desai, and D. Hilton. Systemic nanoparticle paclitaxel (nab-paclitaxel) for in-stent restenosis I (SNAPIST-I): a first-in-human safety and dose-finding study. Clin. Cardiol. 30:165–170, 2007.
Melder, R. J. IN.PACT drug-eluting balloon: technology and pre-clinical findings. EuroPCR., 2012.
Nikol, S., T. Y. Heuehns, and B. Höfling. Molecular biology and post-angioplasty restenosis. Atherosclerosis. 123:17–31, 1996.
Pan, Ch J, J. J. Tang, Y. J. Weng, J. Wang, and N. Huang. Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J. Control. Release 116:42–49, 2006.
Pósa, A., N. Nyolczas, R. Hemetsberger, N. Pavo, O. Petnehazy, Z. Petrasi, G. Sangiorgi, and M. Gyongyosi. Optimization of drug-eluting balloon use for safety and efficacy: evaluation of the 2nd generation paclitaxel-eluting DIOR-balloon in porcine coronary arteries. Catheter. Cardiovasc. Interv. 76:395–403, 2010.
Raval, A., A. Choubey, C. Engineer, H. Kotadia, and D. Kothwala. Novel biodegradable polymeric matrix coated cardiovascular stent for controlled drug delivery. Trends Biomater. Artif. Organs. 20:101–110, 2007.
Raval, A., J. Parikh, and C. Engineer. Mechanism of controlled release kinetics from medical devices. Braz. J. Chem. Eng. 27:211–225, 2010.
Rits, J., J. A. van Herwaarden, A. K. Jahrome, D. Krievins, and F. L. Moll. The incidence of arterial stent fractures with exclusion of coronary, aortic, and non-arterial settings. J. Vasc. Surg. 48:773, 2008.
Scheinert, D., S. Duda, T. Zeller, H. Krankenberg, J. Ricke, M. Bosiers, G. Tepe, S. Naisbitt, and K. Rosenfield. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc. Interv. 7:10–19, 2014.
Scheinert, D., S. Scheinert, J. Sax, C. Piorkowski, S. Braunlich, M. Ulrich, G. Biamino, and A. Schmidt. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J. Am. Coll. Cardiol. 45:312–315, 2005.
Scheller, B. Contrast media as carriers for local drug delivery Successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur. Heart J. 24:1462–1467, 2003.
Scheller, B., U. Speck, C. Abramjuk, U. Bernhardt, M. Bohm, and G. Nickenig. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 110:810–814, 2004.
Scheller, B., U. Speck, B. Romeike, A. Schmitt, M. Sovak, M. Böhm, and H. P. Stoll. Contrast media as carriers for local drug delivery: successful inhibition of neointimal proliferation in the porcine coronary stent model. Eur. Heart J. 24:1462–1467, 2003.
Scheller, B., U. Speck, A. Schmitt, M. Böhm, and G. Nickenig. Addition of paclitaxel to contrast media prevents restenosis after coronary stent implantation. J. Am. Coll. Cardiol. 42:1415–1420, 2003.
Schillinger, M., M. Haumer, G. Schlerka, W. Mlekusch, and M. Exner. Restenosis after percutaneous transluminal angioplasty in the femoropopliteal segment: the role of inflammation. J. Endovasc. Therapy. 8:477–483, 2001.
Schroeder, H., D. R. Meyer, B. Lux, F. Ruecker, M. Martorana, and S. Duda. Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: outcomes from the ILLUMENATE first-in-human study. Catheter. Cardiovasc. Interv. 86:278–286, 2015.
Scientific, B. The next generation of drug-coated balloons. Endovasc. Today Eur. 2, 2015.
Speck, U., N. Stolzenburg, D. Peters, and B. Scheller. How does a drug-coated balloon work? Overview of coating techniques and their impact. J. Cardiovasc. Surg. 51:3–11, 2016.
Sternberg, K., S. Kramer, C. Nischan, N. Grabow, T. Langer, G. Hennighausen, and K. P. Schmitz. In vitro study of drug-eluting stent coatings based on poly(l-lactide) incorporating cyclosporine A: drug release, polymer degradation and mechanical integrity. J. Mater. Sci. 18:1423–1432, 2007.
Stolzenburg, N., J. Breinl, S. Bienek, M. Jaguszewski, M. Lochel, M. Taupitz, U. Speck, S. Wagner, and J. Schnorr. Paclitaxel-coated balloons: investigation of drug transfer in healthy and atherosclerotic arteries—first experimental results in rabbits at low inflation pressure. Cardiovasc. Drugs Ther. 30:263–270, 2016.
Tepe, G., B. Schnorr, T. Albrecht, K. Brechtel, C. D. Claussen, B. Scheller, U. Speck, and T. Zeller. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER Trial. JACC Cardiovasc. Interv. 8:102–108, 2015.
Tepe, G., T. Zeller, T. Albrecht, S. Heller, U. Schwarzwälder, J. Beregi, C. D. Claussen, A. Oldenburg, B. Scheller, and U. Speck. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N. Engl. J. Med. 358:689–699, 2008.
Turner, E. A., A. C. Stenson, and S. K. Yazdani. HPLC-MS/MS method for quantification of paclitaxel from keratin containing samples. J. Pharm. Biomed. Anal. 139:247–251, 2017.
Wang, L. Drug releasing coatings for medical devices., 2016.
Werk, M., T. Albrecht, D. R. Meyer, M. N. Ahmed, A. Behne, U. Dietz, G. Eschenbach, H. Hartmann, C. Lange, B. Schnorr, H. Stiepani, G. B. Zoccai, and E. L. Hanninen. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ. Cardiovasc. Interv. 5:831–840, 2012.
Werk, M., S. Langner, B. Reinkensmeier, H. F. Boettcher, G. Tepe, U. Dietz, N. Hosten, B. Hamm, U. Speck, and J. Ricke. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation 118:1358–1365, 2008.
Yazdani, S. K., E. Pacheco, M. Nakano, F. Otsuka, S. Naisbitt, F. D. Kolodgie, E. Ladich, S. Rousselle, and R. Virmani. Vascular, downstream, and pharmacokinetic responses to treatment with a low dose drug-coated balloon in a swine femoral artery model. Catheter. Cardiovasc. Interv. 83:132–140, 2014.
Zhang, Y. L., J. Bendrick-Peart, T. Strom, M. Haschke, and U. Christians. Development and validation of a high-throughput assay for quantification of the proliferation inhibitor ABT-578 Using LC/LC-MS/MS in blood and tissue samples. Ther. Drug Monit. 27:770–778, 2005.
Funding
This work was supported by the American Heart Association [#15SDG25880000, #16PRE27350003], National Institute of Health [#1R15HL127596] and a research grant from the University of South Alabama Office and Research Development.
Conflict of interest
Saami K. Yazdani serves on the Scientific Advisory Board of Advanced Catheter Therapies and Toray Industries and has received grant support from Advanced Catheter Therapies, Toray Industries and Lutonix, Inc., and is a shareholder in Advanced Catheter Therapies. Other co-authors have no conflict of interest to report.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Baruch Barry Lieber and Ajit P. Yoganathan oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1
Deposition of coating on mounted balloons using air spraying. Supplementary material 1 (MP4 1645 kb)
Rights and permissions
About this article
Cite this article
Turner, E.A., Atigh, M.K., Erwin, M.M. et al. Coating and Pharmacokinetic Evaluation of Air Spray Coated Drug Coated Balloons. Cardiovasc Eng Tech 9, 240–250 (2018). https://doi.org/10.1007/s13239-018-0346-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-018-0346-1