Abstract
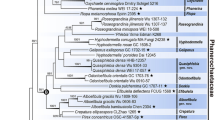
Three genera, Cochliobolus, Bipolaris and Curvularia form a complex that contains many plant pathogens, mostly on grasses (Poaceae) with a worldwide distribution. The taxonomy of this complex is confusing as frequent nomenclatural changes and refinements have occurred. There is no clear morphological boundary between the asexual genera Bipolaris and Curvularia, and some species show intermediate morphology. We investigated this complex based on a set of ex-type cultures and collections from northern Thailand. Combined gene analysis of rDNA ITS (internal transcribed spacer), GPDH (glyceraldehyde 3-phosphate dehydrogenase), LSU (large subunit) and EF1-α (translation elongation factor 1-α) shows that this generic complex divides into two groups. Bipolaris and Cochliobolus species clustered in Group 1 along with their type species, whereas Curvularia species (including species named as Bipolaris, Cochliobolus and Curvularia) clustered in Group 2, with its generic type. The nomenclatural conflict in this complex is resolved giving priority to the more commonly used established generic names Bipolaris and Curvularia. Modern descriptions of the genera Bipolaris and Curvularia are provided and species resolved in this study are transferred to one of these genera based on their phylogeny.




Similar content being viewed by others
References
Alcorn JL (1983) On the genera Cochliobolus and Pseudocochliobolus. Mycotaxon 16:353–379
Agrios GN (2005) Plant pathology, 5th edn. Academic, Amsterdam
Berbee M, Pirseyedi M, Hubbard S (1999) Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 91:964–977
Boedijn KB (1933) Über einige phragmosporen Dematiazen. Bull Jard Bot Buitenzorg 13:120–134
Boonmee SY, Zhang Y, Chomnunti P, Chukeatirote E, Tsui CKM, Bahkali AH, Hyde KD (2011) Revision of lignicolous Tubeufiaceae based on morphological reexamination and phylogenetic analysis. Fungal Divers 53:63–102
Cai L, Hyde K, Taylor P, Weir B, Waller J, Abang M, Zhang J, Yang Y, Phoulivong S, Liu Z (2009) A polyphasic approach for studying Colletotrichum. Fungal Divers 39:183–204
Cai L, Giraud T, Zhang N, Begerow D, Cai G, Shivas RG (2011) The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Divers 50:121–133
Chomnunti P, Schoch CL, Aguirre-Hudson B, Ko-Ko TW, Hongsanan S, Jones EBG, Kodsueb R, Phookamsak R, Chukeatirote E, Bahkali AH (2011) Capnodiaceae. Fungal Divers 51:103–134
Dasgupta SD, Saha A, Saha D (2005) Levels of common antigens in determining pathogenicity of Curvularia eragrostidis in different tea varieties. J Appl Microbiol 98(5):1084–1092
Drechsler C (1934) Phytopathological and taxonomical aspects of Ophilobolus, Pyrenophora, Helminthosporium and a new genus Cochliobolus. Phytopathology 24:953–983
Emami K, Hack E (2002) Conservation of XYN11A and XYN11B xylanase genes in bipolaris sorghicola, cochliobolus sativus, cochliobolus heterostrophus, and cochliobolus spicifer. Curr Microbiol 45:303–306
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microb 61:1323
Goh T (1998) Generic distinction in the Helminthosporium-complex based on restriction analysis of the nuclear ribosomal RNA gene. Fungal Divers 1:109–113
Guo L, Hyde K, Liew E (2000) Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol 147(3):617–630
Hawksworth DL, Crous PW, Redhead SA, Reynolds DR, Samson RA, Seifert KA, Taylor JW, Wingfield MJ (2011) The Amsterdam declaration on fungal nomenclature. IMA Fungus 2:105–112
Hawksworth DL (2012) Managing and coping with names of pleomorphic fungi in a period of transition. Mycosphere 3(2):143–155
Hoog GS de, Guarro J, Gené J, Figueras MJ (eds) (2005) Atlas of Clinical Fungi, CD-ROM version
Hunter GC, Wingfield BD, Crous PW, Wingfield MJ (2006) A multi-gene phylogeny for species of Mycosphaerella occurring on Eucalyptus leaves. Stud Mycol 55:147–161
Hyde K, Chomnunti P, Crous P, Groenewald J, Damm U, Ko TWK, Shivas R, Summerell B, Tan Y (2010) A case for re-inventory of Australia’s plant pathogens. Persoonia 25:50
Katoh K, Asimenos G, Toh H (2009) Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol 537:39–64
Ko Ko TW, Stephenson SL, Bahkali AH, Hyde KD (2011) From morphology to molecular biology: can we use sequence data to identify fungal endophytes? Fungal Divers 36:69–88
Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD, Jeewon R (2006) The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 98:571–583
Liu JK, Phookamsak R, Jones EBG, Zhang Y, Ko Ko TW, Boonmee S, Doilom M, Chukeatirote E, Bahkali AH, Wang Y (2011) Astrosphaeriella is polyphyletic, with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Divers 51:135–154
Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD (2011) Cochliobolus: an overview and current status of species. Fungal Divers 51:3–42
Manamgoda DS, Cai L, Hyde KD, McKenzie EHC, Chukeatirote E (2012) Two new Curvularia species from northern Thailand. (in press)
McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, Marhold K,Nicolson DH, Prado J, Silva PC, Skong JE, Wiersima JH, Turland NJ (2006) International code of botanical nomenclature (Vienna code), Published for IAPT by ARG Gan.
Mendoza L, Ajello L, Taylor JW (2001) The taxonomic status of Lacazia loboi and Rhinosporidium seeberi has been finally resolved with the use of molecular tools. Rev Iberoam Micol 18:95–98
Page RDM (1996) Treeview. An application to display phylogenetic trees on personal computer. Comp Appl Biol Sci 12:357–358
Rinaldi M, Phillips P, Schwartz J, Winn R, Holt G, Shagets F, Elrod J, Nishioka G, Aufdemorte T (1987) Human Curvularia infections: report of five cases and review of the literature. Diagn Micr Infec Dis 6:27–39
Schoch C, Crous PW, Groenewald J, Boehm E, Burgess TI, De Gruyter J, De Hoog G, Dixon L, Grube M, Gueidan C (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Shimizu K, Tanaka C, Peng YL, Tsuda M (1998) Phylogeny of Bipolaris inferred from nucleotide sequences of Brn1, a reductase gene involved in melanin biosynthesis. J Gen Appl Microbiol 44:251–258
Shoemaker RA (1959) Nomenclature of Drechslera and Bipolaris, grass parasites segregated from Helminthosporium. Can J Bot 37:879–887
Sivanesan A (1987) Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. CAB International
Swofford D (2002) PAUP 4.0 b10: phylogenetic analysis using parsimony. Sinauer Associates, Sunderland
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Tsuda M, Ueyama A, Nishihara N (1977) Pseudocochliobolus nisikadoi, the perfect state of Helminthosporium coicis. Mycologia 69:1109–1120
Udayanga D, Liu X, McKenzie EHC, Chukeatirote E, Bahkali AHA, Hyde KD (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers 50:189–225
Valente P, Ramos J, Leoncini O (1999) Sequencing as a tool in yeast molecular taxonomy. Can J Microbiol 45(11):949–958
Wakker DZ (1898) het Suikerriet op Java: 196
Zhang Y, Crous PW, Scoch CL, Hyde KD (2012) Pleosporales. Fungal Divers 53:1–221
Acknowledgements
This study is funded by China NSFC (31110103906) and CAS (KSCX2-YW-Z-1026) and Thailand Research Fund BRG528002. Dimuthu S. Manamgoda thanks the State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing and the Mushroom Research Foundation, Chiang Mai, Thailand for a postgraduate scholarship. Amy Y. Rossman (USDA- ARS) is thanked for the helpful comments on the nomenclatural decisions. Dhanushka Udayanga (Mae Fah Luang University, Chiang Rai) and Fang Liu (CAS, Beijing) are thanked for the assistance.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Manamgoda, D.S., Cai, L., McKenzie, E.H.C. et al. A phylogenetic and taxonomic re-evaluation of the Bipolaris - Cochliobolus - Curvularia Complex. Fungal Diversity 56, 131–144 (2012). https://doi.org/10.1007/s13225-012-0189-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-012-0189-2