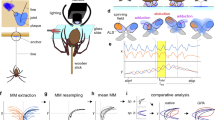
Abstract
The rigid leg segments of arthropods are flexibly connected by joints, which usually consist of two ball-and-bowl hinges, permitting a uniaxial pivoting up to 140°. Here, we report the occurrence of hyperflexible joints (range of movements = 160–200°) in the pedipalps (second pair of appendages) of some harvestmen (Sabaconidae and Nemastomatidae), representing some of the most flexible leg joints among arthropods. Hyperflexion is achieved by a reduction of hinges and a strong constriction of the joint region. We demonstrate that hyperflexion occurs during prey capture and is used to clamp appendages of the prey, in addition to attachment by glue secreted by specialized setae. By means of high-speed video recordings, we found that in the Sabaconidae the tibiotarsal joint of the pedipalp can flex extremely rapidly (<5 ms), limiting prey escape. This is the fastest reported predatory strike in arachnids and caused both by leverage and a click mechanism. By comparative analysis of different related taxa, we retraced joint evolution and found that hyperflexion has independently evolved in Sabaconidae and Nemastomatidae, with totally different joint kinematics. We hypothesize that (rapid) hyperflexion evolved to enhance the efficiency of the pedipalp as a means of prey capture, because in springtails detachable scales limit the action of the sticky secretion of pedipalpal setae.



Similar content being viewed by others
References
Abrams, P. A. (2000). The evolution of predator-prey interactions: theory and evidence. Annual Review of Ecology and Systematics, 31, 79–105.
Alberti, G. (1973). Ernährungsbiologie und Spinnvermögen der Schnabelmilben (Bdellidae, Trombidiformes). Zeitschrift für Morphologie der Tiere, 76(4), 285–338.
Alberti, G. (2010). On predation in Epicriidae (Gamasida, Anactinotrichida) and fine-structural details of their forelegs. Soil Organisms, 82, 179–192.
Ass, M. (1973). Die Fangbeine der Arthropoden, ihre Entstehung, Evolution und Funktion. Deutsche Entomologische Zeitschrift, 20(1‐3), 127–152.
Bauer, T. (1982). Prey-capture in a ground-beetle larva. Animal Behaviour, 30(1), 203–208.
Bauer, T., & Pfeiffer, M. (1991). Shooting springtails with a sticky rod - the flexible hunting behavior of Stenus comma (Coleoptera, Staphylinidae) and the counterstrategies of its prey. Animal Behaviour, 41, 819–828. doi:10.1016/S0003-3472(05)80349-5.
Bauer, T., & Völlenkle, W. (1976). Hochfrequente Filmaufnahmen als Hilfsmittel bei der Analyse von Angriffs- und Fluchtverhalten in einer Räuber-Beute-Beziehung unter Bodentieren (Collembolenfang visuell jagender Carabiden). Wissenschaftlicher Film (Wien), 17, 4–11.
Betz, O., & Kölsch, G. (2004). The role of adhesion in prey capture and predator defence in arthropods. Arthropod Structure & Development, 33(1), 3–30. doi:10.1016/j.asd.2003.10.002.
Christian, E. (1978). Jump of Springtails. Naturwissenschaften, 65(9), 495–496. doi:10.1007/Bf00702849.
Christian, E. (1979). Der Sprung der Collembolen. Zoologische Jahrbücher Physiologie, 83, 457–490.
Christiansen, K., & Pike, E. (2002). Cretaceous Collembola (Arthropoda, Hexapoda) from the Upper Cretaceous of Canada. Cretaceous Research, 23(2), 165–188.
Corrette, B. J. (1990). Prey capture in the praying mantis Tenodera aridifolia sinensis: coordination of the capture sequence and strike movements. Journal of Experimental Biology, 148(1), 147–180.
Dawkins, R., & Krebs, J. R. (1979). Arms races between and within species. Proceedings of the Royal Society of London. Series B: Biological Sciences, 205(1161), 489–511.
Dunlop, J. A. (2006). Baltic amber harvestman types (Arachnida: Opiliones: Eupnoi and Dyspnoi). Fossil Record, 9(2), 167–182.
Eggs, B., Wolff, J. O., Kuhn-Nentwig, L., Gorb, S. N., & Nentwig, W. (2015). Hunting without a web: How lycosoid spiders overwhelm their prey. Ethology, 121(12), 1166–1177.
Giribet, G., & Dunlop, J. A. (2005). First identifiable Mesozoic harvestman (Opiliones: Dyspnoi) from Cretaceous Burmese amber. Proceedings of the Royal Society of London B: Biological Sciences, 272(1567), 1007–1013.
Gorb, S. N. (1999). Evolution of the dragonfly head-arresting system. Proceedings of the Royal Society of London. Series B: Biological Sciences, 266(1418), 525–535.
Groh, S., & Giribet, G. (2014). Polyphyly of Caddoidea, reinstatement of the family Acropsopilionidae in Dyspnoi, and a revised classification system of Palpatores (Arachnida, Opiliones). Cladistics, 31, 277–290.
Gronenberg, W. (1995). The fast mandible strike in the trap-jaw ant Odontomachus. Journal of Comparative Physiology A, 176(3), 399–408.
Gronenberg, W. (1996). Fast actions in small animals: springs and click mechanisms. Journal of Comparative Physiology A, 178(6), 727–734.
Gronenberg, W., Tautz, J., & Hölldobler, B. (1993). Fast trap jaws and giant neurons in the ant Odontomachus. Science, 262(5133), 561–563.
Hintzpeter, U., & Bauer, T. (1986). The antennal setal trap of the ground beetle Loricera pilicornis: a specialization for feeding on Collembola. Journal of Zoology, 208(4), 615–630.
Hopkin, S. P. (1997). Biology of the springtails (Insecta, Collembola). Oxford; New York: Oxford University Press.
Juberthie, C., Lopez, A., & Juberthie-Jupeau, L. (1981). Sur l’équipement adéno-sensoriel du pédipalpe de l’opilion troglophile Sabacon paradoxum Simon (Palpatores, Sabaconidae). In B. F. Back (Ed.), Proceedings VIII International Congress of Speleology (pp. 810–811). Americus: Department of Geology, Georgia Southwestern College.
Maddison, W. P., & Maddison, D. R. (2015). Mesquite: a modular system for evolutionary analysis. Version 3.04. http://mesquiteproject.org .
Murphy, E., & Patek, S. (2012). Strike mechanics of an ambush predator: the spearing mantis shrimp. Journal of Experimental Biology, 215(24), 4374–4384.
Parry, D., & Brown, R. (1959). The hydraulic mechanism of the spider leg. Journal of Experimental Biology, 36(2), 423–433.
Patek, S., & Caldwell, R. (2005). Extreme impact and cavitation forces of a biological hammer: strike forces of the peacock mantis shrimp Odontodactylus scyllarus. Journal of Experimental Biology, 208(19), 3655–3664.
Santer, R. D., & Hebets, E. A. (2009). Prey capture by the whip spider Phrynus marginemaculatus CL Koch. Journal of Arachnology, 37(1), 109–112.
Schomann, A., Afflerbach, K., & Betz, O. (2008). Predatory behaviour of some Central European pselaphine beetles (Coleoptera: Staphylinidae: Pselaphinae) with descriptions of relevant morphological features of their heads. European Journal of Entomology, 105(5), 889–907.
Schönhofer, A. L. (2013). A taxonomic catalogue of the Dyspnoi Hansen and Sørensen, 1904 (Arachnida: Opiliones). Zootaxa, 3679(1), 1–68.
Schönhofer, A. L., McCormack, M., Tsurusaki, N., Martens, J., & Hedin, M. (2013). Molecular phylogeny of the harvestmen genus Sabacon (Arachnida: Opiliones: Dyspnoi) reveals multiple Eocene–Oligocene intercontinental dispersal events in the Holarctic. Molecular Phylogenetics and Evolution, 66(1), 303–315.
Shear, W. A. (1975). The opilionid genera Sabacon and Tomicomerus in America (Opiliones, Troguloidea, Ischyropsalidae). Journal of Arachnology, 3, 5–29.
Shear, W. A. (1986). A cladistic analysis of the opilionid superfamily Ischyropsalidoidea, with description of the new family Ceratolasmatidae, the new genus Acuclavella and four new species. American Museum Novitates, New York City, 2844, 1–29.
Shultz, J. W. (2000). Skeletomuscular anatomy of the harvestman Leiobunum aldrichi (Weed, 1893) (Arachnida: Opiliones: Palpatores) and its evolutionary significance. Zoological Journal of the Linnean Society, 128(4), 401–438.
Simon, E. (1879). Opiliones. In E. Simon (Ed.), Les Arachnides de France. Tome 7. Contenant les ordres des Chernetes, Scorpiones et Opiliones (pp. 116–311). Paris: Librairie Encyclopédique de Roret.
Sudo, S., Shiono, M., Kainuma, T., Shirai, A., & Hayase, T. (2013). The kinematics of jumping of globular springtail. Journal of Aero Aqua Bio-mechanisms, 3(1), 85–91.
Verbeek, N., & Boasson, R. (1993). Relationship between types of prey captured and growth form in Drosera in southwestern Australia. Australian Journal of Ecology, 18(2), 203–207.
Vermeij, G.J. (1994). The evolutionary interaction among species: selection, escalation, and coevolution. Annual Review of Ecology and Systematics, 25, 219–236.
Versluis, M., Schmitz, B., von der Heydt, A., & Lohse, D. (2000). How snapping shrimp snap: through cavitating bubbles. Science, 289(5487), 2114–2117.
Wauthy, G., Leponce, M., Banai, N., Sylin, G., & Lions, J.-C. (1998). The backward jump of a box moss mite. Proceedings of the Royal Society of London B: Biological Sciences, 265(1411), 2235–2242.
Weygoldt, P. (2000). Whip spiders (Chelicerata: Amblypygi): their biology, morphology and systematics. Stenstrup: Apollo Books.
Willemart, R. H., Santer, R. D., Spence, A. J., & Hebets, E. A. (2011). A sticky situation: solifugids (Arachnida, Solifugae) use adhesive organs on their pedipalps for prey capture. Journal of Ethology, 29(1), 177–180. doi:10.1007/s10164-010-0222-4.
Wolff, J. O., Schönhofer, A. L., Schaber, C. F., & Gorb, S. N. (2014). Gluing the ‘unwettable’: soil-dwelling harvestmen use viscoelastic fluids for capturing springtails. Journal of Experimental Biology, 217(19), 3535–3544.
Wolff, J.O., Schönhofer, A.L., Martens, J., Wijnhoven, H., Taylor, C., & Gorb, S.N. (2016). The evolution of pedipalps and glandular hairs as predatory devices in harvestmen (Arachnida, Opiliones). Zoological Journal of the Linnean Society, (online pre-pub). doi:10.1111/zoj.12375.
Wood, H. M., Griswold, C. E., & Gillespie, R. G. (2012). Phylogenetic placement of pelican spiders (Archaeidae, Araneae), with insight into evolution of the “neck” and predatory behaviours of the superfamily Palpimanoidea. Cladistics, 28(6), 598–626.
Zhang, F., Chen, Z., Dong, R.-R., Deharveng, L., Stevens, M. I., Huang, Y.-H., et al. (2014). Molecular phylogeny reveals independent origins of body scales in Entomobryidae (Hexapoda: Collembola). Molecular Phylogenetics and Evolution, 70, 231–239.
Acknowledgments
We thank Siegfried Huber (Mühlhofen, Germany) for providing living nymphs of Sabacon simoni.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Author contributions
J.O.W. and J.M conceived and designed the study. A.L.S. and J.M. assembled the material and literature. J.O.W. performed the experiments and analyzed the data. J.O.W. wrote the paper, and all the other authors equally contributed in the revision.
Funding
This work was supported by the German National Merit Foundation (Studienstiftung des Deutschen Volkes) to J.O.W. and by German Science Foundation (Deutsche Forschungsgemeinschaft, DFG) (μCT Großgeräteantrag) to S.N.G. J.M. thanks the Feldbausch Foundation and Wagner Foundation, both at Fachbereich Biologie of Johannes Gutenberg University Mainz, for the annual research and travel grants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Video 1
Attacks on springtails with snapping movement of the pedipalp tarsus in Sabacon simoni (annotated high-speed videos). (WMV 6327 kb)
Supplementary Video 2
Attacks on springtails with hyperflexion of tibia and tarsus in Mitostoma chrysomelas (annotated high-speed videos). (WMV 6175 kb)
Supplementary Video 3
Comparison of tarsal flexion speed in S. simoni and M. chrysomelas (annotated high-speed videos). (WMV 120 kb)
Rights and permissions
About this article
Cite this article
Wolff, J.O., Martens, J., Schönhofer, A.L. et al. Evolution of hyperflexible joints in sticky prey capture appendages of harvestmen (Arachnida, Opiliones). Org Divers Evol 16, 549–557 (2016). https://doi.org/10.1007/s13127-016-0278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0278-2