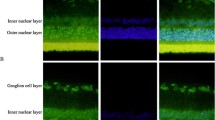
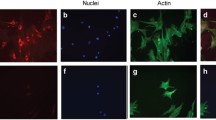
Abstract
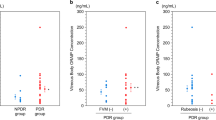
The expression of the proinflammatory cytokine high-mobility group box-1 (HMGB1) is upregulated in epiretinal membranes and vitreous fluid from patients with proliferative diabetic retinopathy (PDR) and in the diabetic retina. We hypothesized that a novel mechanism exists where HMGB1 and NADPH oxidase (Nox)-derived reactive oxygen species (ROS) are mutually enhanced in the diabetic retina, which may be a novel mechanism for promoting upregulation of retinal apoptotic markers induced by diabetes. Vitreous samples from 48 PDR and 34 nondiabetic patients, retinas from 1-month diabetic rats and from normal rats intravitreally injected with HMGB1 and human retinal microvascular endothelial cells (HRMEC) stimulated with HMGB1 were studied by enzyme-linked immunosorbent and spectrophotometric assays, Western blot analysis, RT-PCR, and immunofluorescence. We also studied the effect of the HMGB1 inhibitor glycyrrhizin and apocynin on diabetes-induced biochemical changes in the retinas of rats (n = 5–7 in each groups). HMGB1 and the oxidative stress marker protein carbonyl content levels in the vitreous fluid from PDR patients were significantly higher than in controls (p = 0.021; p = 0.005, respectively). There was a significant positive correlation between vitreous fluid levels of HMGB1 and the levels of protein carbonyl content (r = 0.62, p = 0.001). HMGB1 enhanced interleukin-1β, ROS, Nox2, poly (ADP-ribose) polymerase (PARP)-1, and cleaved caspase-3 production by HRMEC. Diabetes and intravitreal injection of HMGB1 in normal rats induced significant upregulation of ROS, Nox2, PARP-1, and cleaved caspase-3 in the retina. Constant glycyrrhizin and apocynin intake from onset of diabetes did not affect the metabolic status of the diabetic rats, but restored these increased mediators to control values. The results of this study suggest that there is a mutual enhancement between HMGB1 and Nox-derived ROS in the diabetic retina, which may promote diabetes-induced upregulation of retinal apoptotic markers.







Similar content being viewed by others
References
Abu El-Asrar AM, Siddiquei MM, Nawaz MI, Geboes K, Mohammad G (2014) The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediators Inflamm 2014:746415. doi:10.1155/2014/746415
Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA (2010) NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci 30:2967–2978
Al-Shabrawey M, Rojas M, Sanders T, Behzadian A, El-Remessy A, Bartoli M et al (2008) Role of NADPH oxidase in retinal vascular inflammation. Invest Ophthalmol Vis Sci 49:3239–3244
Altomare E, Grattagliano I, Vendemaile G, Micelli-Ferrari T, Signorile A, Cardia L (1997) Oxidative protein damage in human diabetic eye: evidence of a retinal participation. Eur J Clin Invest 27:141–147
Barber A (2003) A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 27:283–290
Burdon RH (1996) Control of cell proliferation by reactive oxygen species. Biochem Soc Trans 24:1028–1032
Cakatay U (2005) Protein oxidation parameters in type 2 diabetic patients with good and poor glycaemic control. Diabetes Metab 31:551–557
Cederlund M, Ghosh F, Arnér K, Andréasson S, Akerström B (2013) Vitreous levels of oxidative stress biomarkers and the radical-scavenger α1-microglobulin/A1M in human rhegmatogenous retinal detachment. Graefes Arch Clin Exp Ophthalmol 251:725–732
Daffu G, del Poz CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM (2013) Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci 14:19891–19910
El-Asrar AM, Missotten L, Geboes K (2011) Expression of high-mobility groups box-1/receptor for advanced glycation end products/osteopontin/early growth response-1 pathway in proliferative vitreoretinal epiretinal membranes. Mol Vis 17:508–518
El-Asrar AM, Nawaz MI, Kangave D, Geboes K, Ola MS, Ahmad S et al (2011) High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Mol Vis 17:1829–1838
Ellis EA, Grant MB, Murray FT, Wachowski MB, Guberski DL, Kubilis PS et al (1998) Increased NADH oxidase activity in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med 24:111–120
Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y et al (2007) Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: role of HMGB1-TLR4 signaling. J Immunol 178:6573–6580
Gao L, Mann GE (2009) Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82:9–20
Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L et al. (2014) Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One 9:e89450. doi:10.1371/journal.pone.0089450
Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51:211–217
Jiang F, Zhang Y, Dusting GJ (2011) NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63:218–242
Kim SW, Jin Y, Shin JH, Kim ID, Lee HK, Park S et al (2012) Glycyrrhizic acid affords robust neuroprotection in the postischemic brain via anti-inflammatory effect by inhibiting HMGB1 phosphorylation and secretion. Neurobiol Dis 46:147–156
Kleniewska P, Piechota A, Skibska B, Goraca A (2012) The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 60:277–294
Kowluru RA, Atasi L, Ho YS (2006) Role of mitochondrial superoxide dismutase in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 47:1594–1599
Kowluru RA, Koppolu P (2002) Diabetes-induced activation of caspase-3 in retina: effect of antioxidant therapy. Free Radic Res 36:993–999
Kowluru RA, Kowluru A, Veluthakal R, Mohammad G, Syed I, Santos JM et al (2014) TIAM1-RAC1 signalling axis-mediated activation of NADPH oxidase-2 initiates mitochondrial damage in the development of diabetic retinopathy. Diabetologia 57:1047–1056
Krynetskaia N, Xie H, Vucetic S, Obradovic Z, Krynetskiy E (2008) High mobility group protein B1 is an activator of apoptotic response to antimetabolite drugs. Mol Pharmacol 73:260–269
Loukovaara S, Koivunen P, Inglés M, Escobar J, Vento M, Andersson S (2014) Elevated protein carbonyl and HIF-1α levels in eyes with proliferative diabetic retinopathy. Acta Ophthalmol 92:323–327
Mohammad G, Siddiquei MM, Abu El-Asrar AM (2013) Poly (ADP-ribose) polymerase mediates diabetes-induced retinal neuropathy. Mediators Inflamm 2013:510451
Mohammad G, Siddiquei MM, Othman A, Al-Shabrawey M, Abu El-Asrar AM (2013) High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Exp Eye Res 107:101–109
Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M et al (2007) Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14:431–441
Ohnishi M, Katsuki H, Fukutomi C, Takahashi M, Motomura M, Fukunaga M et al (2011) HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology 61:975–980
Okuma Y, Liu K, Wake H, Liu R, Nishimura Y, Hui Z et al (2014) Glycyrrhizin inhibits traumatic brain injury by reducing HMGB1-RAGE interaction. Neuropharmacology 85:18–26
Patel P, Zheng G, Bosland MC, Kajdacsy-Balla A, Munirathinam G (2012)The role of high mobility group box 1 (HMGB1) in prostate carcinogenesis. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; Chicago, Illinois. Philadelphia (PA): AACR Abstract nr (535)
Piwowar A, Knapic-Kordecka M, Warwas M (2009) Markers of oxidative protein damage in plasma and urine of type 2 diabetic patients. Br J Biomed Sci 66(8):194–199
Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K et al (2006) Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 203:1637–1642
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Rinnerthaler M, Büttner S, Laun P, Heeren G, Felder TK, Klinger H et al (2012) Yno1p/Aim14p, a NADPH-oxidase ortholog, controls extramitochondrial reactive oxygenspecies generation, apoptosis, and actin cable formation in yeast. Proc Natl Acad Sci U S A 109:8658–8663
Rojas M, Zhang W, Xu Z, Lemtalsi T, Chandler P, Toque HA et al. (2013) Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. PLoS ONE 8:e84357. doi:10.1371/journal.pone.0084357
Santos AR, Dvoriantchikova G1, Li Y1, Mohammad G, Abu El-Asrar AM, Wen R et al. (2014) Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PLoS One 9:e87574. doi:10.1371/journal.pone.0087574
Santos JM, Mohammad G, Zhong Q, Kowluru RA (2011) Diabetic retinopathy, superoxide damage and antioxidants. Curr Pharm Biotechnol 12:352–361
Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K et al (2010) Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53:971–979
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195
Sil R, Ray D, Chakraborti AS (2013) Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J Exp Biol 51:129–138
Simon HU, Haj-Yehia A, Levi-Schaffer F (2000) Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418
Tang D, Kang R, Zen HJ 3rd, Lotze MT (2011) High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 14:1315–1335
Ulloa L, Messmer D (2006) High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev 17:189–201
Wu Y, Zhang K, Zhao L, Guo J, Hu X, Chen Z (2013) Increased serum HMGB1 is related to oxidative stress in patients with atrial fibrillation. J Int Med Res 41:1796–1802
Zheng L, Szabó C, Kern TS (2004) Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes 53:2960–2967
Acknowledgments
The authors thank Ms. Connie B. Unisa-Marfil for secretarial work. This project was funded by National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award number (12-MED2604-02) and Dr. Nasser Al-Rashid Research Chair in Ophthalmology (Abu El-Asrar AM).
Declaration of interest and financial disclosure
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammad, G., Alam, K., Nawaz, M.I. et al. Mutual enhancement between high-mobility group box-1 and NADPH oxidase-derived reactive oxygen species mediates diabetes-induced upregulation of retinal apoptotic markers. J Physiol Biochem 71, 359–372 (2015). https://doi.org/10.1007/s13105-015-0416-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0416-x