Abstract
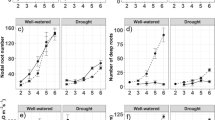
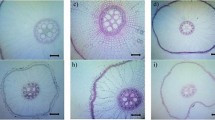
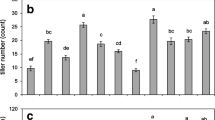
Rice production is oftentimes confronted by environmental constraints such as drought which affects the water uptake of roots. The internal apoplastic barriers in the roots are believed to regulate/constrain the radial water flow through the gaps in between the cells. Thus, in this study, a screenhouse experiment was conducted using four rice cultivars namely, Jasmine, Arabon, Basmati, and UPLRi-3 (drought-tolerant variety), to determine the effects of 3 weeks of drought stress in the common sites of root apoplastic barriers, such as the outer part of the root (OPR) (composed of epidermis, exodermis, and sclerenchymatous layer) and root endodermis, and to identify from these tissues putative responses that may be relevant to drought-tolerance of rice roots. The exodermis was not affected during drought while the epidermis shrank extremely. The second week of drought imposition resulted in a significant increase in exodermal and endodermal cell sizes. Drought enhanced the lignification in the sclerenchymatous layer and suberization in the endodermis. The drought-tolerant variety UPLRi-3 developed the thickest lignification of sclerenchymatous layer (2.15 μm), followed by Jasmine (1.9 μm), and lastly Arabon and Basmati (1.59 and 1.51 μm, respectively). The varieties UPLRi-3, Arabon, and Jasmine produced high suberin deposition in the endodermis, which were not significantly different from each other (3.76, 3.51, and 3.49 μm, respectively), while Basmati produced the lowest (2.81 μm). The control variety UPLRi-3 demonstrated a highly positive root response which can be associated to its drought-tolerance trait. The results obtained suggest rice root’s dynamic coping mechanisms with the effects of drought to protect itself from rapid dying and prevent internal water loss.
Similar content being viewed by others
References
Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M. 2012. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 35(9): 1618–1630
Bouman BAM, Hengsdijk H, Hardy B, Bindraban PS, Tuong TP, Ladha JK, eds. 2002. Water-wise rice production. Proceedings of the International Workshop on Water-wise Rice Production, 8–11 April 2002, Los Baños, Philippines. Los Baños (Philippines): International Rice Research Institute. 356 p
Colmer TD, Gibberd MR, Wiengweera A, Tinh TK. 1998. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J. Exp. Bot. 49(325): 1431–1436
Comas LH, Becker SR, Von Mark VC, Byrne PF, Dierig DA. 2013. Root traits contributing to plant productivity under drought. Front. Plant Sci 4
Dai A, Trenberth KE, Qian T. 2004. A global dataset of Palmer Drought Severity Index for 1870–2002: relationship with soil moisture and effects of surface warming. J. Hydrometeorol. 5(6): 1117–1130
Dos Santos WD, Maria de Lourdes LF, Finger A, Teixeira AC, Ferrarese-Filho O. 2004. Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J. Chem. Ecol. 30(6): 1203–1212
Eissenstat DM, Achor DS. 1999. Anatomical characteristics of roots of citrus rootstocks that vary in specific root length. New Phytol. 141(2): 309–321
Ekanayake IJ, O'toole JC, Garrity DP, Masajo TM. 1985. Inheritance of root characters and their relations to drought resistance in rice. Crop Sci. 25(6): 927–933
Enstone DE, Peterson, CA, Ma F. 2002. Root endodermis and exodermis: structure, function, and responses to the environment. J. Plant Growth Regul. 21(4): 335–351
Gowda VR, Henry A, Yamauchi A, Shashidhar HE, Serraj R. 2011. Root biology and genetic improvement for drought avoidance in rice. Field Crops Res. 122(1): 1–13
Hanson AD, Peacock WJ, Evans LT, Arntzen CJ, Khush GS. 1990. Drought resistance in rice. Nature, 234: 2. In: Development of drought–resistant cultivars using physio-morphological traits in rice. S Fukai, M Cooper, eds, Field Crops Res. 40: 67-86
Henry A, Cal AJ, Batoto TC, Torres RO, Serraj R. 2012. Root attributes affecting water uptake of rice (Oryza sativa) under drought. J. Exp. Bot. 63(13): 4751–4763
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. 2001. The exodermis: a variable apoplastic barrier. J. Exp. Bot. 52(365): 2245–2264
Javot H, Maurel C. 2002. The role of aquaporins in root water uptake. Ann. Bot. 90(3): 301–313
Jeong JS, Kim YS, Redillas MC, Jang G, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim JK. 2013. OsNAC5 overexpression enlarges root diameter in rice plants leading to enhanced drought tolerance and increased grain yield in the field. Plant Biotechnol. J. 11(1): 101–114
Kotula L, Ranathunge K, Schreiber L, Steudle E. 2009. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J. Exp. Bot. 60(7): 2155–2167
Lee DK, Jung H, Jang G, Jeong JS, Kim YS, Ha SH, Do Choi Y, Kim JK. 2016. Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol. pp 00379
Lian HL, Yu X, Ye Q, Ding X S, Kitagawa Y, Kwak SS, Su WA, Tang ZC. 2004. The role of aquaporin RWC3 in drought avoidance in rice. Plant Cell Physiol. 45(4): 481–489
Lynch JP, Chimungu JG, Brown KM. 2014. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J. Exp. Bot. 65(21): 6155–6166
Miyamoto N, Steudle E, Hirasawa T, Lafitte R. 2001. Hydraulic conductivity of rice roots. J. Exp. Bot. 52(362): 1835–1846
Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. 2014. An overview of global rice production, supply, trade, and consumption. Ann. NY Acad. Sci. 1324(1): 7–14
Ranathunge K, Kotula L, Steudle E, Lafitte R. 2004. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. J. Exp. Bot. 55(396): 433–447
Ranathunge K, Lin J, Steudle E, Schreiber L. 2011. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant Cell Environ. 34(8): 1223–1240
Ranathunge K, Steudle E, Lafitte R. 2003. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta, 217(2): 193–205
Rebouillat J, Dievart A, Verdeil JL, Escoute J, Giese G, Breitler JC, Gantet P, Espeout S, Guiderdoni E, Périn C. 2009. Molecular genetics of rice root development. Rice 2(1): 15–34
Redillas MC, Jeong JS, Kim YS, Jung H, Bang SW, Choi YD, Ha SH, Reuzeau C, Kim JK. 2012. The overexpression of OsNAC9 alters the root architecture of rice plants enhancing drought resistance and grain yield under field conditions. Plant Biotechnol. J. 10(7): 792–805
Sauter M. 2013. Root responses to flooding. Curr. Opin. Plant Biol. 16(3): 282–286
Steudle E. 2000. Water uptake by roots: effects of water deficit. J. Exp. Bot. 51(350): 1531–1542
Tuong TP, Bouman BAM. 2003. Rice production in water-scarce environments. Water productivity in agriculture: Limits and opportunities for improvement, 1: 13–42
Wang JW, Kao CH. 2006. Aluminum-inhibited root growth of rice seedlings is mediated through putrescine accumulation. Plant Soil, 288(1-2): 373–381
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cortaga, C.Q., Sebidos, R.F. Drought-Induced Modifications on the Outer Part of the Root (OPR) and Root Endodermis of Selected Rice Genotypes. J. Crop Sci. Biotechnol. 22, 131–138 (2019). https://doi.org/10.1007/s12892-019-0018-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12892-019-0018-0