Abstract
Growth hormone-releasing hormone (GHRH) is a peptide hormone secreted by the hypothalamus that regulates the synthesis and secretion of growth hormone (GH) in the pituitary. The extra-hypothalamic GHRH and its cognate receptors (GHRHR and splice variants) play a mitogenic role by stimulating cell proliferation and preventing apoptotic cell death. It is well established that GHRH antagonists inhibit the growth, tumorigenicity, and metastasis of various human malignancies. In this work, we studied the effect of two new GHRH antagonists, MIA602 and MIA690, on thyroid cancer. We studied the effect of MIA602 and MIA690 on thyroid cancer in vitro, using human thyroid cancer cell lines, and in vivo, using chicken embryo chorioallantoic membrane (CAM) assays. We found that mRNA for GHRH and GHRH receptor is expressed in thyroid cell lines and in samples of thyroid tumors. Immunohistochemistry confirmed the expression of GHRHR protein in specimens of thyroid tumor. We observed that GHRH antagonists inhibited the growth and increased apoptosis of thyroid cancer cells. In vivo, the antagonists inhibited growth and angiogenesis of engrafted thyroid tumors. Our results suggest that GHRH expression may play a role in growth of thyroid cancer and that GHRH antagonists can be a therapeutic option for thyroid cancer patients.
Similar content being viewed by others
Introduction
Thyroid carcinoma is the most frequent endocrine cancer [9, 22] and comprises a group of tumors with different features. It includes papillary thyroid carcinoma (PTC), the most common type, and follicular thyroid carcinoma (FTC), both considered differentiated thyroid carcinomas, usually indolent, with low rates of morbidity and mortality; poorly differentiated thyroid carcinoma (PDTC), with intermediate prognosis, and anaplastic thyroid carcinoma (ATC), the most aggressive and invasive type of thyroid carcinoma and the least responsive to treatment [22].
Growth hormone-releasing hormone (GHRH) is a peptide hormone, secreted by the hypothalamus, which regulates the synthesis and secretion of growth hormone (GH) in the pituitary [18, 19]. GHRH and its receptor, GHRHR, are also expressed in various extra-hypothalamic normal and malignant tissues, immune cells, and cancer cell lines [1, 3, 8, 19, 25]. In normal and neoplastic tissues, the extra-hypothalamic GHRH plays a mitogenic role by stimulating cell proliferation and preventing apoptotic cell death. These effects are mediated through the pituitary type of the GHRHR (pGHRHR) and possibly through some of its four splice variants (SV1–4) [5, 14]. SV1 is the variant that has the highest structural homology with GHRHR and probably is the main splice variant that mediates the effects of GHRH and of GHRH antagonists in tumors [14].
Several hundred peptide analogs were synthesized that exert an antagonistic effect on the GHRHR [19, 25], and their effects on cell growth were tested. GHRH antagonists inhibit the growth, tumorigenicity, and metastasis of various human malignancies, including prostatic, breast, ovarian, endometrial, and brain cancers [19], through blocking the stimulatory effect of tumoral GHRH. This effect can be indirect, by the suppression of the axis between pituitary GH and hepatic insulin-like growth factor (IGF-1), or direct, by the blockade of action of autocrine and/or paracrine GHRH on GHRH receptors on tumors [8, 19]. The targeting of GHRH receptors (GHRHR and SV1) is a potential anticancer therapy, as GHRH antagonists can competitively block the binding of tumoral GHRH to the pGHRHR and its splice variants on tumors [3, 7, 13]. The expression of GHRHR and SV1 had already been reported in normal thyroid and PTC cell lines, and it was observed that treatment with the GHRH antagonist, MIA602, led to a reduction of the tumor invasion marker MMP2, in a PTC cell line [2].
In this study, we evaluated the expression of messenger RNA (mRNA) for GHRH and GHRHR in thyroid cell lines and human papillary thyroid carcinoma samples and the presence of a GHRHR protein by immunohistochemistry in human papillary thyroid carcinoma samples. In addition, we evaluated the effect of the new GHRH antagonists, MIA602 and MIA690, both in vitro, using three human thyroid cancer cell lines, 8505C, C643, and TPC1, by assessing the viability and apoptosis and in vivo, using the chicken embryo chorioallantoic membrane (CAM) assay, and by analyzing tumor growth and angiogenesis.
Materials and Methods
Human Tumor Samples
Thirty-three frozen tissue specimens and 41 paraffin tissue samples of classical papillary thyroid tumors (cPTCs) and follicular variant papillary thyroid tumors (fvPTCs) were collected in Hospital São João, Porto, Portugal. Areas of adjacent non-tumor thyroid tissues (n = 25) were also analyzed. All the procedures involving samples were approved by the Local Ethical Committee and were in accordance with the National Ethical rules and Helsinki declaration.
Quantitative Real-Time PCR
Total RNA was extracted from PTC frozen tissues and cell lines using the Trizol method (Invitrogen) followed by cDNA conversion (Thermo Scientific) according to the manufacturer’s instructions. RNA quality was assessed through β-actin amplification. Quantitative real-time PCR reactions were performed with the TaqMan Real-Time PCR Master Mix (ThermoFisher Scientific), using hGHRH primers (reverse: 5′-GCTCTCCAATTCCATTTGCTTT-3′; forward: 5′-CTGCTCCAGGACATCATGAG -3′; Probe: 5′-/56-FAM/CTCTTGGTT/ZEN/GCTCTCTCCCTGCT/3IABkFQ/-3′); hGHRHR primers (reverse: 5′-CAGCAGCTCCAGAGGCA-3′; forward: 5′-TCTCACTTCAGCTCAGAGTCA-3′; Probe: 5′−/56-FAM/AGGGTAAGG/ZEN/TGGAAAGGGCTCAGA/3IABkFQ/-3′); and human TATA Box-Binding Protein (TBP) primers (reverse: 5′-CAAGAACTTAGCTGGAAAACCC-3′; forward: 5′-GATAAGAGAGCCACGAACCAC-3′; Probe: 5′-/56-FAM/CACAGGAGC/ZEN/CAAGAGTGAAGAACAGT/3IABkFQ/-3′). RNA from human pituitary was used as a positive control. The cycling conditions were as follows: 3 min at 95°, 3 min at 95°, and 1 min at 60° for 45 cycles. Negative control samples were run in each reaction. Quantitative real-time PCR reactions were performed using 7500 Real-Time PCR system (Applied Biosystems). Samples were run in triplicates. The relative quantification of target genes was determined using the ΔΔCT method [11].
Immunohistochemistry
Sections were deparaffinized and rehydrated, followed by heat-induced antigen retrieval procedure in a steamer, with 10 mM sodium citrate buffer at pH 6.0. The sections were incubated overnight at 4 °C in a humidified chamber with the primary antibody GHRHR (1:2000; Abcam, CB4 0FL Cambridge), which detects both full-length GHRHR and SV1 variant. The detection was performed with a labeled streptavidin–biotin immunoperoxidase detection system (Thermo Scientific/Lab Vision, Fremont, USA), and the immunohistochemical staining was developed with 3,3′-diaminobenzidine substrate. Human stomach tissue was used as negative (omission of primary antibody) and positive control. Three observers (P.S., H.P., and T.B.G.) evaluated tumor cell immunoreactivity without the knowledge of any clinical data of the cases. Adjacent non-tumor tissue immunoreactivity for GHRHR was used as an internal control. An IHC score was settled as previously reported [6] and results from the multiplication of the intensity score of the staining (negative = 0, weak = 1, moderate = 2, and strong = 3) and the extension score of the immunohistochemical staining of tumor cells (0–5% = 0, 6–25% = 1, 26–50% = 2, 51–75% = 3, and 76–100% = 4).
Cell Lines and Culture Conditions
The human thyroid cancer cell lines 8505C, C643, hTh74, T238, BCPAP, K1, and TPC1 were maintained in RPMI medium (Gibco/BRL, Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO, Invitrogen, Carlsbad, CA). XTC1 line was maintained in DMEM HAM-F12 medium (Gibco/BRL, Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO, Invitrogen, Carlsbad, CA), 10 μg/mL insulin and 0.01 U/mL TSH. Cell lines were maintained in a humidified atmosphere, with 5% CO2, at 37 °C.
Treatment of Thyroid Cancer Cell Lines with MIA602 and MIA690
The GHRH antagonists, MIA602 and MIA690, were synthesized in the laboratory of one of us (A.V.S.) as previously described [25, 26]. MIA602 and MIA690 were dissolved in DMSO and added to the culture medium, with reduced supplement of fetal bovine serum (1%). Each GHRH antagonist was used at 1 μM concentration for 48 and 72 h of treatment. Thyroid cancer cell lines incubated in culture medium supplemented with 0.1% DMSO, served as controls.
Cell Viability Assay
The effect of MIA602 or MIA690 on the viability of thyroid cancer cell lines was analyzed by Presto Blue (PB) assay. Cells were seeded in 96-well plates at a density of 3 × 103 (C643) or 5 × 103 (8505C and TPC1) in 200 μL medium. After 24 h, the medium was replaced by medium containing either MIA602 or MIA690 at the concentration range of 0.1–10 μM. Cells were then incubated for 24 or 48 h, washed with PBS, pH 7.4, and assayed for cell growth using PB according to the manufacturer’s instructions. The fluorescence was measured using a microplate reader (Synergy HT Multi-Mode Microplate Reader, BioTek Instruments Inc., Winooski, VT, USA) at excitation and emission wavelengths of 560 and 590 nm, respectively. The absorbance of the wells containing culture medium and tumor cells only were used as control, and each experimental condition was evaluated in triplicate and repeated twice.
TUNEL Assay
Cells were plated in six-well plates at a final density of 7.5 × 104 (C643) or 5 × 104 (TPC1 and 8505C) cells/well and incubated at 37 °C for 24 h. Cells were then treated with 1 μM concentration of MIA602 or MIA690. As controls, cells were incubated with the vehicle compound alone (0.1% DMSO). Forty-eight and seventy-two hours after treatment, cytospin preparations of all cells (floating and attached) were collected. Cytospin preparations were fixed with 4% paraformaldehyde at room temperature, washed in PBS, and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate on ice. TUNEL analysis was performed using the “in situ cell death detection kit, fluorescein” from Roche® (Roche, Germany), following the manufacturer’s instructions. The proportion of TUNEL-positive (apoptotic) nuclei was determined by counting at least 500 cells. Each experimental condition was evaluated in triplicate and repeated twice.
Western Blot Analysis and Antibodies
Forty-eight and seventy-two hours after treatment with GHRH antagonists, cells were lysed for 15 min at 4 °C using RIPA buffer (1% NP-40 in 150 mM NaCl, 50 mM Tris (pH 7.5), 2 mM EDTA) containing phosphatase and protease inhibitors. Proteins were quantified using a modified Bradford assay (BioRad). Protein samples (40 μg) were separated in 10% SDS/PAGE gels and electroblotted to Hybond ECL membrane (Amersham Biosciences). GHRHR antibody, detecting both full-length GHRHR and SV1 variant (1:3000; Abcam) and peroxidase-conjugated-secondary antibody (Santa Cruz Biotechnology) were used. Specific-protein bands were visualized by the ECL detection solution. Membranes were re-stained with a goat polyclonal antiactin (Santa Cruz Biotechnology) or a mouse monoclonal antitubulin (Sigma) for loading protein control. All experiments and quantifications (using BioRad Quantity One 1-D Analysis software (4.6.6 version)) were performed in triplicate.
In Vivo Chicken Embryo CAM Tumorigenesis and Angiogenic Assay
Briefly, fertilized chicken (Gallus gallus) eggs, with ascertained embryonic day (E), were obtained from commercial sources and incubated horizontally at 37.8° in a humidified atmosphere. On day E3, a square window was opened on the shell after removal of 1.5–2 mL of albumin to allow detachment of the developing CAM. This window was sealed with transparent adhesive tape, and the eggs were returned to the incubator. On day E10, C643 cells were re-suspended in complete medium containing 1 μM concentration of MIA602 or MIA690, or 0.1% DMSO (2 × 106 cells per egg), and were placed on the top of growing CAM using a 3-mm silicon ring under sterile conditions. The eggs were re-sealed and returned to the incubator for 2 days. The treatment was repeated after 48 h. The eggs were incubated for an additional 2 days. The embryos were then euthanized by adding 2 mL of fixative in the top of the CAM. After removing the ring, the CAM was excised from the embryos, photographed ex ovo under a stereomicroscope, at ×20 magnification (Olympus, SZX16 coupled with a DP71 camera). The number of new vessels (less than 20 mm diameter) growing radially towards the inoculation area was counted by a blinded observer. The area of CAM tumors was determined using the Cell A (Olympus) software. Each experimental condition was evaluated in ten eggs and repeated three times. The experiments were done according to European Directive 2010/63/EU and Portuguese law on animal welfare.
Statistical Analysis
Statistical analysis was performed using GraphPadPrism5.0 (GraphPad Software, Inc., La Jolla, CA). The data from the cell line experiments were analyzed by the nonparametric Kruskal-Wallis test and comparisons of two groups were performed by Mann-Whitney test. In the evaluation of the results of CAM assays, ANOVA test was used to calculate the significance at 95% confidence level and multiple-comparison corrections were performed using the post hoc Dunnett’s test. A p value < 0.05 was considered statistically significant.
Results
Expression of mRNA for GHRH and GHRHR in PTC
To verify the expression of GHRH and GHRHR in thyroid cell lines and human thyroid carcinomas, the mRNA expression levels were evaluated by qRT-PCR in thyroid cell lines and PTCs. The expression of mRNA for GHRH and GHRHR was found in a high percentage of the eight thyroid cell lines analyzed (Table 1). The expression of mRNA for GHRH was found in 14/32 (44%) of PTC samples and 10/24 (42%) of adjacent thyroid samples. The expression of mRNA for GHRHR was present in 14/31 (45%) of PTC samples and 9/14 (64%) of adjacent thyroid samples (Table 2; Supplementary Table 1). No significant differences in the expression of mRNA for GHRH and GHRHR were observed between PTCs and adjacent thyroid (data not shown). The expression of mRNA for GHRH was present in most of the patients older than 45 years; in male patients, the expression of mRNA for GHRH was generally below the detection limit, as well as in patients with lymph node metastasis. Similarly, the expression of mRNA for GHRHR was in most cases below the detection limit in patients with lymph node metastasis and also in patients with multiple tumors. The expression of mRNA for both GHRH and GHRHR was mostly below the detection limit in patients with RET/PTC rearrangements (Table 2; Supplementary Table 1).
Immunohistochemical Expression of GHRHR in PTC
Adjacent thyroid tissue had a weak immunohistochemical staining of GHRHR (Fig. 1). Cytoplasmic immunohistochemical expression of GHRHR was observed in 31/41 (76%) of the PTC cases (Fig. 1; Supplementary Table 1). The immunohistochemical expression of GHRHR was positively correlated with the expression of mRNA for GHRHR, although it was not statistically significant (95% CI, −0.10–0.65; p = 0.13). The majority of tumors with ≤ 2 cm presented immunohistochemical expression of GHRHR, as well as all the tumors without lymph node metastasis and the majority of tumors with lymphocytic infiltrate (Table 2; Supplementary Table 1).
Representative microphotographs of immunohistochemical staining for GHRHR expression observed in a case of a classical papillary thyroid tumor (cPTC) (left) and the corresponding adjacent thyroid tissue (right), b a case of follicular variant papillary thyroid tumor (fvPTC) (left) and the corresponding adjacent thyroid tissue (right). DAB × 200 (a, b)
Effects of MIA602 or MIA690 on the Viability of Thyroid Cell Lines
C643, 8505C, and TPC1 thyroid cell lines were exposed to increasing concentrations of MIA602 or MIA690, and the effects on cell viability were evaluated using the PrestoBlue assay. Treatment with GHRH antagonist reduced the viability in a dose-dependent manner after 24 h (results not shown) and 48 h treatment (Fig. 2) in C643 and 8505C. No effect on the viability was observed in TPC1 cells at the maximum concentration of either GHRH antagonist (Fig. 2). The IC50 values were estimated as 3.6 μM for C643 and 1.8 μM for 8505C after 48 h of treatment with MIA602 and 2.5 μM for C643 and 2.0 μM for 8505C after 48 h of treatment with MIA690. The IC50 values for TPC1 were not reached for either of the two antagonists (Fig. 2).
The percentage of cell growth inhibition of C643, 8505C, and TPC1 thyroid cancer cell lines treated with various concentrations up to 10 μM of MIA602 (a) and MIA690 (b) for 48 h, determined by Presto Blue assay. For 8505C cell line, the maximum concentration used was 2 μM of both MIA602 and MIA690. The black line marks the IC50 values obtained. The data are presented as mean ± SD. *p < 0.05, significant differences between cells treated with MIA602 or MIA690 and untreated cells
Effects of MIA602 or MIA690 on Apoptosis of Thyroid Cell Lines
To evaluate the effects of GHRH antagonists on apoptosis, TUNEL assay was performed after 48 and 72 h of treatment with 1 μM of MIA602 or MIA690, in each of the three thyroid cell lines. MIA602 treatment induced 3.8-, 1.9-, and 1.6-fold increase of the number of apoptotic cells in C643, 8505C, and TPC1 lines, respectively, after 48 h (p = 0.01, p = 0.01, and not significant, respectively) and 2.6-, 1.2-, and 2.2-fold after 72 h (p = 0.04, p = 0.04, and p = 0.03, respectively) of treatment (Fig. 3a, b). The same tendency was observed after treatment with MIA690. There was 0.5-, 0.5-, and 1-fold increase of the number of apoptotic cells in C643, 8505C, and TPC1, respectively, after 48 h and 0.9-, 0.4-, and 1.5-fold increase of the number of apoptotic cells in C643, 8505C, and TPC1, respectively, after 72 h, although statistical significance was only reached in TPC1 at 72 h (p = 0.03) (Fig. 3c, d).
The mean percentage of apoptotic cells in C643, 8505C, and TPC1 thyroid cancer cell lines treated with 1 μM MIA602 for 48 h (a) and 72 h (b) and 1 μM MIA690 for 48 h (c) and 72 h (d), determined by TUNEL assay. The data are presented as mean ± SD. *p < 0.05, significant differences between cells treated with MIA602 or MIA690 and untreated cells
Effects of Treatment with MIA602 or MIA690 on GHRHR and SV1 Protein Expression in Thyroid Cell Lines
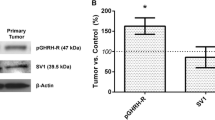
The expression of GHRHR and the splice variant type 1 (SV1) in basal conditions was observed by Western blot analysis in all the three cell lines, with similar levels of expression for GHRHR; a higher expression of SV1 was found in 8505C than in C643 and TPC1 cells in basal conditions.
After treatment with MIA602, a decreased expression of GHRHR (p = 0.01 at 48 h; not significant at 72 h) was observed in C643 cell line, compared with untreated cells. An increase in GHRHR and SV1 expression was found in 8505C line at 48 h (p = 0.01 and p = 0.03, respectively) and at 72 h (p = 0.02 and p = 0.01, respectively). In TPC1 cells, GHRHR and SV1 expression levels also increased (p = 0.01 at 48 h and not significant at 72 h) (Fig. 4a–d).
a, b, e, f Representative Western blot analysis of GHRHR and splice variant (SV1) observed in thyroid cancer cell lines treated with 1 μM MIA602 for 48 h (a) and 72 h (b) and after treatment with 1 μM MIA690 for 48 h (e) and 72 h (f). c, d, g, h Mean protein expression level observed for GHRHR (47 kDa) and SV1 (39.5 kDa) after treatment with 1 μM MIA602 for 48 h (c) and 72 h (d) and 1 μM MIA690 for 48 h (g) and 72 h (h). The data are presented as mean ± SD. *p < 0.05, significant differences between cells treated with 1 μM MIA602 or MIA690 for 48 and 72 h and untreated cells. The images in (a), (b), (e), and (f) are from cropped blots. Vertically sliced images with juxtaposed lanes that were non-adjacent on the gel are separated by a white line. The samples derived from the same experiment and the gels/blots were processed in parallel
After treatment with MIA690, C643 cells showed a significantly decrease of GHRHR expression (p = 0.03 at 48 and 72 h), and no change in SV1 expression, in comparison with untreated cells. An increase in GHRHR and SV1 expression was found in 8505C (p = 0.01 at 48 h and not significant at 72 h), and no significant alterations were observed in TPC1 cells (Fig. 4e–h).
Effects of MIA602 or MIA690 on In Vivo Tumorigenesis and Angiogenesis
To shed light on the mechanism of antitumor action of GHRH antagonists in thyroid cancer, the tumorigenic and the angiogenic responses of C643 thyroid cancer cells were evaluated using the chicken embryo chorioallantoic membrane (CAM) assay. Untreated cells and cells treated with MIA602 or MIA690 were inoculated into the CAM. Treatment with MIA602 and MIA690 was repeated on CAM tumors after 48 h, and the experiment was ended 96 h after inoculation. Tumor sizes were evaluated for each condition in three independent experiments, including a large number of replicas, in order to diminish the variability associated to the in vivo experiments: control (23 eggs), MIA602 (26 eggs), and MIA690 (26 eggs). When possible, the angiogenic responses were also quantified for each condition: control (11 eggs), MIA602 (19 eggs), and MIA690 (13 eggs). A significant decrease in tumor size was observed after treatment with MIA602 (p = 0.02) when compared with the tumors formed by untreated cells, and a non-significant decrease was observed after MIA690 treatment (Fig. 5a, b). In addition, a significant decrease in the number of vessels was observed in the CAM tumors treated with MIA690 (p = 0.01) when compared with the untreated CAM tumors, whereas a non-significant decrease was observed after MIA602 treatment (Fig. 5a, c).
a Representative microphotographs of the CAM xenografts and vascular branches obtained 4 days after inoculation of C643 thyroid cancer cell line untreated and treated with 1 μM MIA602 or MIA690, into the chicken embryo chorioallantoic membrane (CAM). b Mean CAM tumor size obtained after inoculation of C643 treated with 1 μM MIA602 (n = 26 eggs) or MIA690 (n = 26 eggs), compared with untreated cells (n = 23 eggs) (dashed lines in (a)). c Mean number of newly formed vessels obtained after inoculation of C643 treated with 1 μM MIA602 (n = 19 eggs) or MIA690 (n = 13 eggs), compared with untreated cells (n = 11 eggs) (arrows in (a)). The data are presented as mean ± SD. *p < 0.05, significant differences between inoculated cells treated with 1 μM MIA602 or MIA690 and untreated cells
Discussion
GHRH antagonists have been already reported to affect some of the signaling mechanism involved in cell proliferation, survival, and metastasis, through an indirect effect (inhibition of pituitary GH/IGF1 hepatic axis) and/or a direct effect (autocrine/paracrine blockage) on GHRH receptors [19]. Catanuto et al. [2] described the presence of GHRHR and SV1 proteins in PTC samples and proposed that either receptor could be a potential target for anticancer therapy [19, 21]. The same authors [2] also suggested that the presence of these GHRH receptors could indicate a potential for response to the effect of GHRH antagonist in the treatment of thyroid carcinoma. However, this hypothesis and the mechanism of action of GHRH antagonists in thyroid cells lines and tumors remain to be fully clarified. In this study, we established, for the first time, that GHRH antagonists inhibit the growth of thyroid cancer cells, both in vitro and in vivo.
The expression of GHRH and GHRHR at mRNA and protein levels in thyroid cell lines and PTC, in agreement with results of Catanuto et al.’s [2], led us to evaluate the effect of treatment with the new GHRH antagonists MIA602 and MIA690 on thyroid cancer cell lines. Inhibition of GHRH receptors has been shown to reduce proliferation and increase apoptosis in several cancer types [8, 16, 20, 23,24,25]. Our results show that the growth of thyroid cancer cells seems to depend, at least in part, on GHRHR signaling, as GHRH antagonists decreased cell growth and increased apoptosis of thyroid cancer cell lines. It was reported that in prostate and breast cancers, the effect of GHRH antagonist on tumor growth involves the inhibition of PI3K/AKT and RAF/MEK/ERK pathways [16, 23]. A similar mechanism may be operating in thyroid cancer, where both pathways are activated and interconnected, as reported by our group and others [4, 12, 17].
We observed that treatment with GHRH antagonists led to different effects on the expression of GHRHR and SV1 in the three thyroid cancer cell lines. A decrease in GHRHR and SV1 expression was observed in C643 and an increase in 8505C and no change in TPC1 were noticed after treatment with MIA602 or MIA690. These variations seem to be independent of the basal levels of expression of these proteins, which are higher in TPC1 and 8505C comparing with C643. The variation in GHRHR and SV1 expression observed in these cell lines upon treatment does not exclude the sensitivity to the antagonists, as we observed in treated cells, and also as reported in other tumor models [10, 16, 25].
The effect of the antagonist treatment in GHRHR and SV1 expression is not clear, and variable results have been published between groups. Specifically in thyroid, Catanuto et al. [2] observed that tumor cells exposed to MIA602 demonstrated a greater expression of GHRHR and SV1, in agreement with our results in 8505C and TPC1. Kovacs et al. [10] reported that both JV163 and MZJ7138 antagonists upregulated the GHRHR and downregulated the SV1 expression, and they suggest that negative signals by the full-length GHRHR might suppress SV1 expression, which results in inhibition of tumor cell proliferation. Different effects (decreased, increased, or no alterations in GHRHR and SV1 expression) between MIA313, MIA602, MIA604, and MIA610 antagonists, where reported by Klukovits et al. [8]. All the analogs that were used in the study were powerful antagonists of GHRH but led to different effects. Others [15] did not detect significant changes in protein levels of GHRHR and SV1 in PC-3 tumor xenografts after JMR132 treatment. In summary, it seems that the variations in GHRHR and SV1 expression under antagonist treatment are cell line/tissue dependent and are not a direct readout of the sensitivity of the cells to the antagonist.
The expression of mRNA for GHRH and GHRHR in thyroid cancer cell lines seems to be independent of the presence of MAPK alterations and is similar to what we observed in the PTCs in which no association between BRAF or NRAS mutations and GHRHR immunohistochemical expression was observed. However, we noted a low expression of mRNA for GHRH and GHRHR (below the detection limit) as well as low immunohistochemical scores in the PTC cases with RET/PTC rearrangements (Supplementary Table 1). Curiously, the expression of mRNA for GHRHR was below the detection limit in the PTC cell line TPC1 (that also harbors RET/PTC rearrangement). TPC1 was less sensitive to treatment with GHRH antagonist, with only minor decrease in growth. Taken together, these results can imply that patients with thyroid tumors harboring RET/PTC rearrangement could be less sensitive to treatment with GHRH antagonists.
Due to the limited number of PTCs analyzed in our study, we cannot elaborate on a possible association between GHRH and GHRHR expression and clinicopathological features of PTCs. We are aware that our series is small and mainly composed by primary tumors and that further studies in larger series with advanced (metastatic) tumors are necessary in order to clarify this issue.
The in vivo CAM assay was performed with C643 cell line, which presented detectable levels of expression of mRNA of GHRH and GHRHR and was very sensitive to GHRH antagonists. In accordance with the in vitro results, we observed that both antagonists diminish the tumor size, although statistical significance was only reached for MIA602. In addition, the effect of the antagonists on the angiogenic response of thyroid cancer cells was evaluated for the first time and a decrease in the angiogenic response was observed, notably (and statistically significant) for MIA690. Our results support the hypothesis that the antitumor effect of GHRH antagonists can be due, at least in part, to the inhibition of angiogenesis. This mechanism had been reported for ovarian cancer, in which a decrease of VEGF production, after antagonist treatment, was observed [8].
In conclusion, our results support the efficacy of GHRH antagonists in inhibiting thyroid cancer cells growth both in vitro and in vivo and suggest a potential therapeutic application of GHRH antagonists in thyroid cancer patients.
References
Busto R, Schally AV, Varga JL, Garcia-Fernandez MO, Groot K, Armatis P, Szepeshazi K (2002) The expression of growth hormone-releasing hormone (GHRH) and splice variants of its receptor in human gastroenteropancreatic carcinomas. Proc Natl Acad Sci U S A 99:11866–11871
Catanuto P, Tashiro J, Rick FG, Sanchez P, Solorzano CC, Glassberg MK, Block NL, Lew JI, Elliot SJ, Schally AV (2015) Expression of receptors for pituitary-type growth hormone-releasing hormone (pGHRH-R) in human papillary thyroid cancer cells: effects of GHRH antagonists on matrix Metalloproteinase-2. Horm Cancer 6:100–106
Fahrenholtz CD, Rick FG, Garcia MI, Zarandi M, Cai RZ, Block NL, Schally AV, Burnstein KL (2014) Preclinical efficacy of growth hormone-releasing hormone antagonists for androgen-dependent and castration-resistant human prostate cancer. Proc Natl Acad Sci U S A 111:1084–1089
Faustino A, Couto JP, Populo H, Rocha AS, Pardal F, Cameselle-Teijeiro JM, Lopes JM, Sobrinho-Simoes M, Soares P (2012) mTOR pathway overactivation in BRAF mutated papillary thyroid carcinoma. J Clin Endocrinol Metab 97:E1139–E1149
Havt A, Schally AV, Halmos G, Varga JL, Toller GL, Horvath JE, Szepeshazi K, Koster F, Kovitz K, Groot K, Zarandi M, Kanashiro CA (2005) The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci U S A 102:17424–17429
Hohla F, Moder A, Mayrhauser U, Hauser-Kronberger C, Schally AV, Varga JL, Zarandi M, Buchholz S, Huber R, Aigner E, Ritter M, Datz C (2008) Differential expression of GHRH receptor and its splice variant 1 in human normal and malignant mucosa of the oesophagus and colon. Int J Oncol 33:137–143
Kiaris H, Chatzistamou I, Schally AV, Halmos G, Varga JL, Koutselini H, Kalofoutis A (2003) Ligand-dependent and -independent effects of splice variant 1 of growth hormone-releasing hormone receptor. Proc Natl Acad Sci U S A 100:9512–9517
Klukovits A, Schally AV, Szalontay L, Vidaurre I, Papadia A, Zarandi M, Varga JL, Block NL, Halmos G (2012) Novel antagonists of growth hormone-releasing hormone inhibit growth and vascularization of human experimental ovarian cancers. Cancer 118:670–680
Kondo T, Ezzat S, Asa SL (2006) Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 6:292–306
Kovacs M, Schally AV, Hohla F, Rick FG, Pozsgai E, Szalontay L, Varga JL, Zarandi M (2010) A correlation of endocrine and anticancer effects of some antagonists of GHRH. Peptides 31:1839–1846
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
Miyagi E, Braga-Basaria M, Hardy E, Vasko V, Burman KD, Jhiang S, Saji M, Ringel MD (2004) Chronic expression of RET/PTC 3 enhances basal and insulin-stimulated PI3 kinase/AKT signaling and increases IRS-2 expression in FRTL-5 thyroid cells. Mol Carcinog 41:98–107
Qin YJ, Chan SO, Chong KK, Li BF, Ng TK, Yip YW, Chen H, Zhang M, Block NL, Cheung HS, Schally AV, Pang CP (2014) Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation. Proc Natl Acad Sci U S A 111:18303–18308
Rekasi Z, Czompoly T, Schally AV, Halmos G (2000) Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci U S A 97:10561–10566
Rick FG, Schally AV, Block NL, Nadji M, Szepeshazi K, Zarandi M, Vidaurre I, Perez R, Halmos G, Szalontay L (2011) Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci U S A 108:3755–3760
Rick FG, Schally AV, Szalontay L, Block NL, Szepeshazi K, Nadji M, Zarandi M, Hohla F, Buchholz S, Seitz S (2012) Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases. Proc Natl Acad Sci U S A 109:1655–1660
Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J (1997) Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89:457–467
Schally AV, Steelman SL, Bowers CY (1965) Effect of hypothalamic extracts on release of growth hormone in vitro. Proc Soc Exp Biol Med. Soc Exp Biol Med 119:208–212
Schally AV, Varga JL, Engel JB (2008) Antagonists of growth-hormone-releasing hormone: an emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab 4:33–43
Siejka A, Barabutis N, Schally AV (2011) GHRH antagonist MZ-5-156 increases the expression of AMPK in A549 lung cancer cells. Cell Cycle 10:3714–3718
Siejka A, Lawnicka H, Melen-Mucha G, Motylewska E, Komorowski J, Stepien H (2012) Antineoplastic action of growth hormone-releasing hormone (GHRH) antagonists. Recent Pat Anti-cancer Drug Discov 7:56–63
Sipos JA, Mazzaferri EL (2010) Thyroid cancer epidemiology and prognostic variables. Clin Oncol 22:395–404
Siriwardana G, Bradford A, Coy D, Zeitler P (2006) Autocrine/paracrine regulation of breast cancer cell proliferation by growth hormone releasing hormone via Ras, Raf, and mitogen-activated protein kinase. Mol Endocrinol 20:2010–2019
Szalontay L, Schally AV, Popovics P, Vidaurre I, Krishan A, Zarandi M, Cai RZ, Klukovits A, Block NL, Rick FG (2014) Novel GHRH antagonists suppress the growth of human malignant melanoma by restoring nuclear p27 function. Cell Cycle 13:2790–2797
Zarandi M, Cai R, Kovacs M, Popovics P, Szalontay L, Cui T, Sha W, Jaszberenyi M, Varga J, Zhang X, Block NL, Rick FG, Halmos G, Schally AV (2017) Synthesis and structure-activity studies on novel analogs of human growth hormone releasing hormone (GHRH) with enhanced inhibitory activities on tumor growth. Peptides 89:60–70
Zarandi M, Horvath JE, Halmos G, Pinski J, Nagy A, Groot K, Rekasi Z, Schally AV (1994) Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci U S A 91:12298–12302
Acknowledgments
This study was supported by the Portuguese Foundation for Science and Technology through a Post-Doc grant to HP (ref.: SFRH/BPD/85249/2012). IPATIMUP integrates the i3S Research Unit, which is partially supported by FCT. This work was financed by Fundo Europeu de Desenvolvimento Regional (FEDER) funds through the COMPETE 2020-Operacional Programme for Competitiveness and Internationalization (POCI), Portugal 2020, and by Portuguese funds through Fundação para a Ciência e a Tecnologia (FCT)/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). Further funding was obtained from the project “Advancing cancer research: from basic knowledgment to application”;NORTE-01-0145-FEDER-000029; “Projetos Estruturados de I&D&I,” funded by Norte 2020-Programa Operacional Regional do Norte. A.V.S. was supported by the Medical Research Service of the Department of Veterans Affairs and Department of Medicine, Division of Hematology-Oncology and Sylvester Comprehensive Cancer Center, University of Miami, School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest
Electronic Supplementary Material
Supplementary Table 1
(XLSX 19 kb)
Rights and permissions
About this article
Cite this article
Pópulo, H., Nunes, B., Sampaio, C. et al. Inhibitory Effects of Antagonists of Growth Hormone-Releasing Hormone (GHRH) in Thyroid Cancer. HORM CANC 8, 314–324 (2017). https://doi.org/10.1007/s12672-017-0307-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-017-0307-4