Abstract
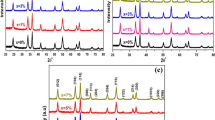
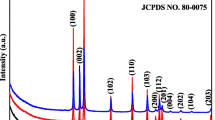
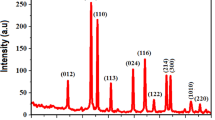
A nanostructured material is a newly emerging field in healthcare environment application because it exhibits strong antibacterial activity by preventing bacterial growth as bacteria are gradually becoming antibiotic resistant. In this article, a potential candidate of antibacterial material, molybdenum trioxide (MoO3), having two phases: the hexagonal and orthorhombic nanocrystals, were successfully synthesized by facile hydrothermal method. Then, the structural, morphological, functional, and optical properties of both h-MoO3 nanorods and α-MoO3 nanoplates were characterized by X-ray diffractometer (XRD), field emission scanning electron microscope (FESEM), Fourier-transform infrared spectroscopy (FTIR), and UV-Vis-NIR spectrophotometer, respectively. XRD patterns revealed hexagonal to orthorhombic phase transition after annealing at 450 °C for 2 h, supported by FTIR spectra. The micrograph analysis by FESEM also confirmed the 1D hexagonal nanorod structure collapsed and converted into two-dimensional (2D) plate-like orthorhombic structure after annealing. The crystallite size and optical bandgap increased from 35 to 135 nm and 2.83 to 2.87 eV, respectively, after phase transformation. The antibacterial activities of both samples were assessed against two Gram-positive bacteria viz. Bacillus subtilis and Staphylococcus aureus and two Gram-negative viz. Escherichia coli and Salmonella enteritidis by agar well diffusion method. The hexagonal nanorods exhibited more activity compared with nanoplates. It was found that the antibacterial activity of the nanoparticles decreased with increasing crystallite size.






Similar content being viewed by others
References
Krishnamoorthy, K., Veerapandian, M., Yun, K., & Jae, S. (2013). New function of molybdenum trioxide nanoplates : toxicity towards pathogenic bacteria through membrane stress. Colloids and Surfaces B: Biointerfaces, 112, 521–524. https://doi.org/10.1016/j.colsurfb.2013.08.026.
Krishnamoorthy, K., & Premanathan, M. (2014). Nanostructured molybdenum oxide-based antibacterial paint: effective growth inhibition of various pathogenic bacteria. Nanotechnology, 315101(25), 10pp. https://doi.org/10.1088/0957-4484/25/31/315101.
Poole, K. (2001). Multidrug resistance in Gram-negative bacteria. Current Opinion in Microbiology, 4(5), 500–508. https://doi.org/10.1016/S1369-5274(00)00242-3.
Krishnamoorthy, K., Moon, J. Y., Hyun, H. B., Cho, S. K., & Kim, S. J. (2012). Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. Journal of Materials Chemistry, 22(47), 24610–24617. https://doi.org/10.1039/c2jm35087d.
Moritz, M., & Geszke-moritz, M. (2013). The newest achievements in synthesis , immobilization and practical applications of antibacterial nanoparticles. Chemical Engineering Journal, 228, 596–613. https://doi.org/10.1016/j.cej.2013.05.046.
Holtz, R. D., Lima, B. A., Souza Filho, A. G., Brocchi, M., & Alves, O. L. (2012). Nanostructured silver vanadate as a promising antibacterial additive to water-based paints. Nanomedicine: Nanotechnology, Biology, and Medicine, 8(6), 935–940. https://doi.org/10.1016/j.nano.2011.11.012.
Raghupathi, K. R., Koodali, R. T., & Manna, A. C. (2011). Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir, 27(7), 4020–4028. https://doi.org/10.1021/la104825u.
Das, D., Nath, B. C., Phukon, P., & Dolui, S. K. (2013). Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids and Surfaces B: Biointerfaces, 101, 430–433. https://doi.org/10.1016/j.colsurfb.2012.07.002.
Li, Y., Yu, H., Huang, X., Wu, Z., & Chen, M. (2017). A simple synthesis method to prepare a molybdenum oxide hole-transporting layer for efficient polymer solar cells. RSC Advances, 7(13), 7890–7900. https://doi.org/10.1039/c7ra00303j.
Dagar, J., Tyagi, P., Ahmad, R., Singh, R., Sinha, O. P., Suman, C. K., & Srivastava, R. (2015). Application of 2D-MoO3 nano-flakes in organic light emitting diodes: effect of semiconductor to metal transition with irradiation. RSC Advances, 5(11), 8397–8403. https://doi.org/10.1039/C4RA12430H.
Zhou, L., Yang, L., Yuan, P., Zou, J., Wu, Y., & Yu, C. (2010). α-MoO3 nanobelts: a high performance cathode material for lithium ion batteries. The Journal of Physical Chemistry C, 114(49), 21868–21872. https://doi.org/10.1021/jp108778v.
Cauduro, A. L. F., Dos Reis, R., Chen, G., Schmid, A. K., Méthivier, C., Rubahn, H. G., et al. (2017). Crystalline molybdenum oxide thin-films for application as interfacial layers in optoelectronic devices. ACS Applied Materials and Interfaces, 9(8), 7717–7724. https://doi.org/10.1021/acsami.6b14228.
Ranjba, M., Delalat, F., & Salamati, H. (2017). Molybdenum oxide nanosheets prepared by an anodizing-exfoliation process and observation of photochromic properties. Applied Surface Science, 396, 1752–1759. https://doi.org/10.1016/j.apsusc.2016.11.225.
Ji, F., Ren, X., Zheng, X., Liu, Y., Pang, L., Jiang, J., & Liu, S. (Frank). (2016). 2D-MoO3 nanosheets for superior gas sensors. Nanoscale, 8(16), 8696–8703. https://doi.org/10.1039/C6NR00880A.
Prakash, N. G., Dhananjaya, M., Narayana, A. L., Shaik, D. P., Rosaiah, P., & Hussain, O. M. (2018). High performance one dimensional α-MoO3 nanorods for supercapacitor applications. Ceramics International, 44(8), 9967–9975. https://doi.org/10.1016/j.ceramint.2018.03.032.
Hu, H., Deng, C., Xu, J., Zhang, K., & Sun, M. (2015). Metastable h -MoO3 and stable α -MoO3 microstructures: controllable synthesis, growth mechanism and their enhanced photocatalytic activity. Journal of Experimental Nanoscience, 10(17), 1336–1346. https://doi.org/10.1080/17458080.2015.1012654.
Andersson, G., & Magnéli, A. (1950). On the crystal structure of molybdenum trioxide. Acta Chemica Scandinavica, 4, 793–797. https://doi.org/10.3891/acta.chem.scand.04-0793.
McCarron, E. M. (1986). β-MoO 3 : a metastable analogue of WO3. Journal of the Chemical Society, Chemical Communications, 101(4), 336–338. https://doi.org/10.1039/C39860000336.
Zhou, J., Xu, N. S., Deng, S. Z., Chen, J., She, J. C., & Wang, Z. L. (2003). Large-area nanowire arrays of molybdenum and molybdenum oxides: synthesis and field emission properties. Advanced Materials, 15(21), 1835–1840. https://doi.org/10.1002/adma.200305528.
Ramana, C. V., Atuchin, V. V., Troitskaia, I. B., Gromilov, S. A., Kostrovsky, V. G., & Saupe, G. B. (2009). Low-temperature synthesis of morphology controlled metastable hexagonal molybdenum trioxide (MoO3). Solid State Communications, 149(1–2), 6–9. https://doi.org/10.1016/j.ssc.2008.10.036.
Deki, S., Béléké, A. B., Kotani, Y., & Mizuhata, M. (2009). Liquid phase deposition synthesis of hexagonal molybdenum trioxide thin films. Journal of Solid State Chemistry, 182(9), 2362–2367. https://doi.org/10.1016/j.jssc.2009.06.033.
Song, J., Ni, X., Gao, L., & Zheng, H. (2007). Synthesis of metastable h-MoO3 by simple chemical precipitation. Materials Chemistry and Physics, 102(2–3), 245–248. https://doi.org/10.1016/j.matchemphys.2006.12.011.
Sayede, A. D., Amriou, T., Pernisek, M., Khelifa, B., & Mathieu, C. (2005). An ab initio LAPW study of the α and β phases of bulk molybdenum trioxide, MoO3. Chemical Physics, 316(1–3), 72–82. https://doi.org/10.1016/j.chemphys.2005.04.036.
Rakkesh, R. A., & Balakumar, S. (2015). Morphology dependent photocatalytic activity of α-MoO3 nanostructures towards mutagenic acridine orange dye. Journal of Nanoscience and Nanotechnology, 15(6), 4316–4324. https://doi.org/10.1166/jnn.2015.9723.
Dong, W., & Dunn, B. (1998). Sol–gel synthesis and characterization of molybdenum oxide gels. Journal of Non-Crystalline Solids, 225, 135–140. https://doi.org/10.1016/S0022-3093(98)00018-0.
Parviz, D., Kazemeini, M., Rashidi, A. M., & Jafari Jozani, K. (2010). Synthesis and characterization of MoO3 nanostructures by solution combustion method employing morphology and size control. Journal of Nanoparticle Research, 12(4), 1509–1521. https://doi.org/10.1007/s11051-009-9727-6.
Cai, L., Rao, P. M., & Zheng, X. (2011). Morphology-controlled flame synthesis of single, branched, and flower-like α-MoO3 nanobelt arrays. Nano Letters, 11(2), 872–877. https://doi.org/10.1021/nl104270u.
Zeng, H. C. (1998). Chemical etching of molybdenum trioxide: a new tailor-made synthesis of MoO3 catalysts. Inorganic Chemistry, 37(3), 1967–1973. https://doi.org/10.1021/Ic971269v.
Klinbumrung, A., Thongtem, T., & Thongtem, S. (2012). Characterization of orthorhombic α-MoO3 microplates produced by a microwave plasma process. Journal of Nanomaterials, 2012. https://doi.org/10.1155/2012/930763.
Kim, H.-U., Son, J., Kulkarni, A., Ahn, C., Kim, K. S., Shin, D., et al. (2017). Highly uniform wafer-scale synthesis of α -MoO3 by plasma enhanced chemical vapor deposition. Nanotechnology, 28(17), 175601. https://doi.org/10.1088/1361-6528/aa67d1.
Song, L. X., Xia, J., Dang, Z., Yang, J., Wang, L. B., & Chen, J. (2012). Formation, structure and physical properties of a series of α-MoO3 nanocrystals: from 3D to 1D and 2D. CrystEngComm, 14(8), 2675. https://doi.org/10.1039/c2ce06567c.
Fakhri, A., & Nejad, P. A. (2016). Antimicrobial, antioxidant and cytotoxic effect of molybdenum trioxide nanoparticles and application of this for degradation of ketamine under different light illumination. Journal of Photochemistry and Photobiology B: Biology, 159, 211–217. https://doi.org/10.1016/j.jphotobiol.2016.04.002.
Desai, N., & Mali, S. (2015). Chemically grown MoO3 nanorods for antibacterial activity study. Journal of Nanomedicine & Nanotechnology, 06(06). https://doi.org/10.4172/2157-7439.1000338.
Shafaei, S., Dörrstein, J., Guggenbichler, J. P., & Zollfrank, C. (2017). Cellulose acetate-based composites with antimicrobial properties from embedded molybdenum trioxide particles. Letters in Applied Microbiology, 64(1), 43–50. https://doi.org/10.1111/lam.12670.
Zollfrank, C., Gutbrod, K., Wechsler, P., & Guggenbichler, J. P. (2012). Antimicrobial activity of transition metal acid MoO3 prevents microbial growth on material surfaces. Materials Science and Engineering C, 32(1), 47–54. https://doi.org/10.1016/j.msec.2011.09.010.
Shafaei, S., Van Opdenbosch, D., Fey, T., Koch, M., Kraus, T., Guggenbichler, J. P., & Zollfrank, C. (2016). Enhancement of the antimicrobial properties of orthorhombic molybdenum trioxide by thermal induced fracturing of the hydrates. Materials Science and Engineering C, 58, 1064–1070. https://doi.org/10.1016/j.msec.2015.09.069.
Kumar, A., & Pandey, G. (2017). Synthesis , characterization , effect of temperature on band gap energy of molybdenum oxide nano rods and their antibacterial activity. American Journal of Nanosciences, 3(4), 81–85. https://doi.org/10.11648/j.ajn.20170304.12.
Krishnamoorthy, K., Premanathan, M., Veerapandian, M., & Jae Kim, S. (2014). Nanostructured molybdenum oxide-based antibacterial paint: Effective growth inhibition of various pathogenic bacteria. Nanotechnology, 25(31). https://doi.org/10.1088/0957-4484/25/31/315101.
Kothaplamoottil, S., Akshay, S., Vinod, K. K. P., & Rajendra, V. T. P. (2019). Greener assembling of MoO3 nanoparticles supported on gum arabic: cytotoxic effects and catalytic efficacy towards reduction of p - nitrophenol. Clean Technologies and Environmental Policy. https://doi.org/10.1007/s10098-019-01726-9.
Angamuthuraj, C., Rajeswari Yogamalar, N., & A. C. B. (2016). Hydrothermally synthesized h-MoO3 and α-MoO3 nanocrystals: new findings on crystal structure dependent charge transport. Crystal Growth & Design, 16(4), 1984–1995. https://doi.org/10.1021/acs.cgd.5b01571.
Chithambararaj, A., Sanjini, N. S., Velmathi, S., & Chandra Bose, A. (2013). Preparation of h-MoO3 and α-MoO3 nanocrystals: comparative study on photocatalytic degradation of methylene blue under visible light irradiation. Physical Chemistry Chemical Physics, 15(35), 14761. https://doi.org/10.1039/c3cp51796a.
Wongkrua, P., Thongtem, T., & Thongtem, S. (2013). Synthesis of h- and α -MoO3 by refluxing and calcination combination: phase and morphology transformation, photocatalysis, and photosensitization. Journal of Nanomaterials, 2013. https://doi.org/10.1155/2013/702679.
Sen, S. K., Noor, M., Al Mamun, M. A., Manir, M. S., Matin, M. A., Hakim, M. A., et al. (2019). An investigation of 60Co gamma radiation-induced effects on the properties of nanostructured α-MoO3 for the application in optoelectronic and photonic devices. Optical and Quantum Electronics, 51(3), 82. https://doi.org/10.1007/s11082-019-1797-9.
Badr, A. M., Elshaikh, H. A., & Afify, H. H. (2017). Hydrothermal synthesis and influence of later heat treatment on the structural evolution , optical and electrical properties of nanostructured α -MoO3 single crystals. Journal of Physics D: Applied Physics, 50, 505111 (13pp). doi:https://doi.org/10.1088/1361-6463/aa97aa.
Chithambararaj, A., Winston, B., Sanjini, N. S., Velmathi, S., & Bose, A. C. (2015). Band gap tuning of h-MoO3 nanocrystals for efficient visible light photocatalytic activity against methylene blue dye. Journal of Nanoscience and Nanotechnology, 15(7), 4913–4919. https://doi.org/10.1166/jnn.2015.9846.
Hossain, M. K., Mortuza, A. A., Sen, S. K., Basher, M. K., Ashraf, M. W., Tayyaba, S., et al. (2018). A comparative study on the influence of pure anatase and Degussa-P25 TiO2 nanomaterials on the structural and optical properties of DSSC photoanode. Optik, 171(March), 507–516. https://doi.org/10.1016/j.ijleo.2018.05.032.
Jangade, P., Arjunwadkar, P. R., & Nagarbawadi, M. A. (2016). Structural characterization of lead titanate ( PbTiO3 ) sample using FULLPROF. IOSR Journal of Applied Physics, 8(6), 57–60. https://doi.org/10.9790/4861-0806055760.
Po, B. (2013). Rietveld refinement and ionic conductivity of Ca8.4Bi1.6(PO4)6O1.8. Journal of Solid State Chemistry, 197, 154–159. https://doi.org/10.1016/j.jssc.2012.08.005.
Bih, H., Saadoune, I., Bih, L., Mansori, M., Toufik, H., Fuess, H., & Ehrenberg, H. (2016). Synthesis, Rietveld refinements, infrared and Raman spectroscopy studies of the sodium diphosphate NaCryFe1-yP2O7 (0 ≤ y ≤ 1). Journal of Molecular Structure, 1103, 103–109. https://doi.org/10.1016/j.molstruc.2015.09.014.
Lemine, O. M., Bououdina, M., Sajieddine, M., Al-Saie, A. M., Shafi, M., Khatab, A., et al. (2011). Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Physica B: Condensed Matter, 406(10), 1989–1994. https://doi.org/10.1016/j.physb.2011.02.072.
Sahai, A., Goswami, N., Mishra, M., & Gupta, G. (2018). Structural, vibrational and electronic properties of CuO nanoparticles synthesized via exploding wire technique. Ceramics International, 44(2), 2478–2484. https://doi.org/10.1016/j.ceramint.2017.10.224.
Mendelson, M. I. (1969). Average grain size in polycrystalline ceramics. Journal of American Ceramic Soceity, 52(111), 443–446. https://doi.org/10.1111/j.1151-2916.1969.tb11975.x.
Shafaei, S., Lackner, M., Meier, M., Plank, J., Guggenbichler, J. P., & C. Z. (2013). Polymorphs of molybdenum trioxide as innovative antimicrobial materials. Surface Innovations, 1(SI4), 202–208. https://doi.org/10.1680/si.13.00021.
Muraoka, Y., Grenier, J.-C., Petit, S., & Pouchard, M. (1999). Preparation of hexagonal MoO3 by “Chimie Douce” reaction with NO2. Solid State Sciences, 1(2–3), 133–148. https://doi.org/10.1016/S1293-2558(00)80070-9.
Xia, T., Li, Q., Liu, X., Meng, J., & Cao, X. (2006). Morphology-controllable synthesis and characterization of single-crystal molybdenum trioxide. Journal of Physical Chemistry B, 110(5), 2006–2012. https://doi.org/10.1021/jp055945n.
Zakharova, G. S., Täschner, C., Volkov, V. L., Hellmann, I., Klingeler, R., Leonhardt, A., & Büchner, B. (2007). MoO3-δ nanorods: synthesis, characterization and magnetic properties. Solid State Sciences, 9(11), 1028–1032. https://doi.org/10.1016/j.solidstatesciences.2007.07.022.
Chen, Y., Lu, C., Xu, L., Ma, Y., Hou, W., & Zhu, J. J. (2010). Single-crystalline orthorhombic molybdenum oxide nanobelts: synthesis and photocatalytic properties. CrystEngComm, 12(11), 3740–3747. https://doi.org/10.1039/c000744g.
Chithambararaj, A., & Bose, A. C. (2011). Hydrothermal synthesis of hexagonal and orthorhombic MoO3 nanoparticles. Journal of Alloys and Compounds, 509(31), 8105–8110. https://doi.org/10.1016/j.jallcom.2011.05.067.
Sen, S. K., Chandra, T., Manir, M. S., Dutta, S., Hossain, M. N., & Podder, J. (2019). Effect of Fe-doping and post annealing temperature on the structural and optical properties of MoO 3nanosheets (pp. 1–13). Journal of Materials Science: Materials in Electronics. https://doi.org/10.1007/s10854-019-01805-z.
Chithambararaj, A., & Chandra Bose, A. (2014). Role of synthesis variables on controlled nucleation and growth of hexagonal molybdenum oxide nanocrystals: investigation on thermal and optical properties. CrystEngComm, 16(27), 6175–6186. https://doi.org/10.1039/c4ce00418c.
Shore, K. A. (2014). Electronic processes in non-crystalline materials (Second Edition), by N.F. Mott and E.A. Davis. Contemporary Physics, 55(4), 337–337. doi:https://doi.org/10.1080/00107514.2014.933254.
Azam, A., & Oves, M. (2012). Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. International Journal of Nanomedicine, 7, 3527–3535. https://doi.org/10.2147/IJN.S29020.
Nin, Æ. N. (2008). Synthesis and antibacterial activity of silver nanoparticles with different sizes. Journal of Nanoparticle Research, 10, 1343–1348. https://doi.org/10.1007/s11051-008-9428-6.
Acknowledgments
We would like to thank Dr. M, A. Hakim of Department of Glass and Ceramic Engineering and Dr. Md. Kamruzzaman Pramanik, Microbiology and Industrial Irradiation Division, for their cordial supports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Humans and Animals Statement
None.
Informed Consent
None.
Funding Statement
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sen, S.K., Dutta, S., Khan, M.R. et al. Characterization and Antibacterial Activity Study of Hydrothermally Synthesized h-MoO3 Nanorods and α-MoO3 Nanoplates. BioNanoSci. 9, 873–882 (2019). https://doi.org/10.1007/s12668-019-00671-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-019-00671-7