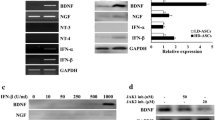
Abstract
Huntington disease (HD) is an inherited disorder hallmarked by progressive deterioration of specific neurons, followed by movement and cognitive anomalies. Cell therapy approaches in neurodegenerative conditions have concentrated on the replenishment of lost/dying neurons with functional ones. Multipotent mesenchymal stem cells (MSCs) have been represented as a potential remedy for HD. In this study, we evaluated the in vitro and in vivo efficacy of umbilical cord matrix stem cells (UCMSCs) and their paracrine effect against oxidative stress with a specific focus on HD. To this end, UCMSCs were isolated, immunophenotypically characterized by the positive expression of MSC markers, and exhibited multilineage potentiality. Besides, synthesis of neurotrophic factors of GDNF and VEGF by UCMSC was confirmed. Initially, PC12 cells were exposed to superoxide in the presence of conditioned media (CM) collected from UCMSC (UCMSC-CM) and cell viability plus neuritogenesis were measured. Next, bilateral striatal transplantation of UCMSC in 3-nitropropionic acid (3-NP) lesioned rat models was conducted, and 1 month later, post-graft analysis was performed. According to our in vitro results, CM of UCMSC protected PC12 cells against oxidative stress and considerably enhanced cell viability and neurite outgrowth. On the other hand, transplanted UCMSC survived, decreased gliosis, and ameliorated motor coordination and muscle activity, along with an increase in striatal volume as well as in dendritic length of the striatum in HD rats. Collectively, our findings imply that UCMSCs provide an enriched platform by largely their paracrine factors, which downgrades the unfavorable effects of oxidative stress.









Similar content being viewed by others
References
Aliaghaei A, Gardaneh M, Maghsoudi N, Salehinejad P, Gharib E (2016) Dopaminergic induction of umbilical cord mesenchymal stem cells by conditioned medium of choroid plexus epithelial cells reduces Apomorphine-induced rotation in parkinsonian rats. Arch Iran Med 19
Bantubungi K, Blum D, Cuvelier L, Wislet-Gendebien S, Rogister B, Brouillet E, Schiffmann SN (2008) Stem cell factor and mesenchymal and neural stem cell transplantation in a rat model of Huntington's disease. Mol Cell Neurosci 37:454–470
Boroujeni ME, Gardaneh M (2017) Umbilical cord: an unlimited source of cells differentiable towards dopaminergic neurons. Neural Regen Res 12:1186
Boroujeni M, Gowda P, Johnson J, Rao J, Saremy S (2012) The proliferation and differentiation capacity of bone marrow derived-human mesenchymal stem cells in early and late doubling. Asian J Biochem 7:27–36
Boroujeni ME, Gardaneh M, Shahriari MH, Aliaghaei A, Hasani S (2017) Synergy between choroid plexus epithelial cell-conditioned medium and knockout serum replacement converts human adipose-derived stem cells to dopamine-secreting neurons. Rejuvenation Res
Chao KC, Chao KF, Fu YS, Liu SH (2008) Islet-like clusters derived from mesenchymal stem cells in Wharton's jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One 3:e1451
Clelland CD, Barker RA, Watts C (2008) Cell therapy in Huntington disease. Neurosurg Focus 24:E9
Dey ND, Bombard MC, Roland BP, Davidson S, Lu M, Rossignol J, Sandstrom MI, Skeel RL, Lescaudron L, Dunbar GL (2010) Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington's disease. Behav Brain Res 214:193–200
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Ellison SM, Trabalza A, Tisato V, Pazarentzos E, Lee S, Papadaki V, Goniotaki D, Morgan S, Mirzaei N, Mazarakis ND (2013) Dose-dependent neuroprotection of VEGF165 in Huntington's disease striatum. Mol Ther 21:1862–1875
Fan C-G, Zhang Q-j, Zhou J-r (2011) Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev 7:195–207
Fink KD, Rossignol J, Crane AT, Davis KK, Bombard MC, Bavar AM, Clerc S, Lowrance SA, Song C, Lescaudron L (2013) Transplantation of umbilical cord-derived mesenchymal stem cells into the striata of R6/2 mice: behavioral and neuropathological analysis. Stem Cell Res Ther 4:130
Hsiao ST-F, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, Dilley RJ (2011) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 21:2189–2203
Jendro M, Goronzy JJ, Weyand CM (1991) Structural and functional characterization of HLA-DR molecules circulating in the serum. Autoimmunity 8:289–296
Kells AP, Fong DM, Dragunow M, During MJ, Young D, Connor B (2004) AAV-mediated gene delivery of BDNF or GDNF is neuroprotective in a model of Huntington disease. Mol Ther 9:682–688
Kerkis I, Haddad MS, Valverde CW, Glosman S (2015) Neural and mesenchymal stem cells in animal models of Huntington’s disease: past experiences and future challenges. Stem Cell Res Ther 6:232
Kim HO, Choi S-M, Kim H-S (2013) Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Eng Regen Med 10:93–101
Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, Zimprich C, Aebischer P, Svendsen CN, Suzuki M (2013) Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol Ther 21:1602–1610
Kumar P, Kalonia H, Kumar A (2010) Huntington’s disease: pathogenesis to animal models. Pharmacol Rep 62:1–14
Lee H, Lee JK, Min WK, Bae JH, He X, Schuchman EH, Bae JS, Jin HK (2010) Bone marrow-derived mesenchymal stem cells prevent the loss of Niemann-pick type C mouse Purkinje neurons by correcting sphingolipid metabolism and increasing Sphingosine-1-phosphate. Stem Cells 28:821–831
Lin Y-T, Chern Y, Shen C-KJ, Wen H-L, Chang Y-C, Li H, Cheng T-H, Hsieh-Li HM (2011) Human mesenchymal stem cells prolong survival and ameliorate motor deficit through trophic support in Huntington's disease mouse models. PLoS One 6:e22924
Machado CV, Telles PDS, Nascimento ILO (2013) Immunological characteristics of mesenchymal stem cells. Rev Bras Hematol Hemoter 35:62–67
McBride JL, During MJ, Wuu J, Chen E-Y, Leurgans SE, Kordower JH (2003) Structural and functional neuroprotection in a rat model of Huntington’s disease by viral gene transfer of GDNF. Exp Neurol 181:213–223
McBride JL, Ramaswamy S, Gasmi M, Bartus RT, Herzog CD, Brandon EP, Zhou L, Pitzer MR, Berry-Kravis EM, Kordower JH (2006) Viral delivery of glial cell line-derived neurotrophic factor improves behavior and protects striatal neurons in a mouse model of Huntington’s disease. Proc Natl Acad Sci 103:9345–9350
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54
Myers RH, Vonsattel JP, Paskevich PA, Kiely DK, Stevens TJ, Cupples LA, Richardson EP Jr, Bird ED (1991) Decreased neuronal and increased oligodendroglial densities in Huntington's disease caudate nucleus. J Neuropathol Exp Neurol 50:729–742
Noorafshan A, Abdollahifar M-A, Asadi-Golshan R, Rashidian-Rashidabadi A, Karbalay-Doust S (2014) Curcumin and sertraline prevent the reduction of the number of neurons and glial cells and the volume of rats’ medial prefrontal cortex induced by stress. Acta Neurobiol Exp 74:44–53
Noorafshan A, Abdollahifar M-A, Karbalay-Doust S, Asadi-Golshan R, Rashidian-Rashidabadi A (2015) Sertraline and curcumin prevent stress-induced morphological changes of dendrites and neurons in the medial prefrontal cortex of rats. Folia Neuropathol 53:69–79
Olson SD, Kambal A, Pollock K, Mitchell G-M, Stewart H, Kalomoiris S, Cary W, Nacey C, Pepper K, Nolta JA (2012) Examination of mesenchymal stem cell-mediated RNAi transfer to Huntington's disease affected neuronal cells for reduction of huntingtin. Mol Cell Neurosci 49:271–281
Peterson AL, Nutt JG (2008) Treatment of Parkinson’s disease with trophic factors. Neurotherapeutics 5:270–280
Pineda J, Rubio N, Akerud P, Urban N, Badimon L, Arenas E, Alberch J, Blanco J, Canals J (2007) Neuroprotection by GDNF-secreting stem cells in a Huntington's disease model: optical neuroimage tracking of brain-grafted cells. Gene Ther 14:118–128
Pouladi MA, Morton AJ, Hayden MR (2013) Choosing an animal model for the study of Huntington's disease. Nat Rev Neurosci 14:708–721
Ramaswamy S, McBride JL, Kordower JH (2007) Animal models of Huntington's disease. ILAR J 48:356–373
Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS (2014) Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol 10:204–216
Rossignol J, Boyer C, Lévèque X, Fink KD, Thinard R, Blanchard F, Dunbar GL, Lescaudron L (2011) Mesenchymal stem cell transplantation and DMEM administration in a 3NP rat model of Huntington's disease: morphological and behavioral outcomes. Behav Brain Res 217:369–378
Sadan O, Shemesh N, Barzilay R, Dadon-Nahum M, Blumenfeld-Katzir T, Assaf Y, Yeshurun M, Djaldetti R, Cohen Y, Melamed E (2012) Mesenchymal stem cells induced to secrete neurotrophic factors attenuate quinolinic acid toxicity: a potential therapy for Huntington's disease. Exp Neurol 234:417–427
Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MDF, Jazedje T, Okamoto OK, Muotri AR, Zatz M (2008) Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells 26:146–150
Snyder BR, Chiu AM, Prockop DJ, Chan AW (2010) Human multipotent stromal cells (MSCs) increase neurogenesis and decrease atrophy of the striatum in a transgenic mouse model for Huntington's disease. PLoS One 5:e9347
Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA (2003) VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest 111:1843–1851
Túnez I, Tasset I, Pérez-De La Cruz V, Santamaría A (2010) 3-Nitropropionic acid as a tool to study the mechanisms involved in Huntington’s disease: past, present and future. Molecules 15:878–916
Volm M, Mattern J, Koomägi R (1999) Inverse correlation between apoptotic (Fas ligand, caspase-3) and angiogenic factors (VEGF, microvessel density) in squamous cell lung carcinomas. Anticancer Res 19:1669–1671
Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel J-PG, Faull RL (2014) The neuropathology of Huntington’s disease. Behavioral Neurobiology of Huntington's Disease and Parkinson's Disease. Springer, In, pp 33–80
Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC (2004) Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells 22:1330–1337
Acknowledgements
The present research work was supported by Vice Chancellor of Neuroscience Reserch Center of Shahid Beheshti University of Medical Sciences and this project is part of M.D. thesis of MJ. Ebrahimi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Ethics Committee of the University approved this animal experiment (IR SBMU.PHNS.REC.1395.7).
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Fig. 1
The site of transplanted cells in the striatum. The implanted cells stained with hematoxylin were injected in the striatum and were still detectable after one month. The dashed line showed the site of injection. Magnification: 10X. (DOCX 281 kb).
ESM 1
The stats for rotarod test performance over the 4-week period. (XLSX 14 kb).
Rights and permissions
About this article
Cite this article
Ebrahimi, M.J., Aliaghaei, A., Boroujeni, M.E. et al. Human Umbilical Cord Matrix Stem Cells Reverse Oxidative Stress-Induced Cell Death and Ameliorate Motor Function and Striatal Atrophy in Rat Model of Huntington Disease. Neurotox Res 34, 273–284 (2018). https://doi.org/10.1007/s12640-018-9884-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-018-9884-4