Abstract
Purpose
Risk to healthcare workers treating asymptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the operating room depends on multiple factors. This review examines the evidence for asymptomatic or pre-symptomatic carriage of SARS-CoV-2, the risk of transmission from asymptomatic patients, and the specific risks associated with aerosol-generating procedures. Protective measures, such as minimization of aerosols and use of personal protective equipment in the setting of treating asymptomatic patients, are also reviewed.
Source
We examined the published literature as well as Societal guidelines.
Principal findings
There is evidence that a proportion of those infected with SARS-CoV-2 have detectable viral loads prior to exhibiting symptoms, or without ever developing symptoms. The degree of risk of transmission from asymptomatic patients to healthcare providers will depend on the prevalence of disease in the population, which is difficult to assess without widespread population screening. Aerosol-generating procedures increase the odds of viral transmission from infected symptomatic patients to healthcare providers, but transmission from asymptomatic patients has not been reported. Techniques to minimize aerosolization and appropriate personal protective equipment may help reduce the risk to healthcare workers in the operating room. Some societal guidelines recommend the use of airborne precautions during aerosol-generating procedures on asymptomatic patients during the coronavirus disease pandemic, although evidence supporting this practice is limited.
Conclusion
Viral transmission from patients exhibiting no symptoms in the operating room is plausible and efforts to reduce risk to healthcare providers include reducing aerosolization and wearing appropriate personal protective equipment, the feasibility of which will vary based on geographic risk and equipment availability.
Résumé
Objectif
Le risque encouru par les travailleurs de la santé traitant des patients asymptomatiques infectés par le syndrome respiratoire aigu sévère du coronavirus 2 (SARS-CoV-2) en salle d’opération dépend de plusieurs facteurs. Ce compte rendu examine les données probantes concernant la présence asymptomatique ou pré-symptomatique du SARS-CoV-2, le risque de transmission des patients asymptomatiques, et les risques spécifiques associés aux interventions générant des aérosols. Nous passons également en revue différentes mesures de protection, telles que la minimisation des aérosols et l’utilisation d’équipements de protection individuelle, dans un contexte de traitement de patients asymptomatiques.
Source
Nous avons examiné la littérature publiée ainsi que les directives sociétales.
Constatations principales
Selon certaines données probantes, une proportion des personnes infectées par le SARS-CoV-2 possèdent des charges virales détectables avant la présence de symptômes, voire même sans manifestation de symptômes. Le degré de risque de transmission des patients asymptomatiques aux travailleurs de la santé dépendra de la prévalence de la maladie dans la population, une donnée difficile à évaluer sans dépistage généralisé. Les interventions générant des aérosols augmentent le risque de transmission virale des patients symptomatiques infectés aux travailleurs de la santé, mais la transmission de patients asymptomatiques n’a pas été rapportée. Les techniques visant à minimiser l’aérosolisation et les équipements de protection individuelle adaptés pourraient être utiles pour réduire le risque des travailleurs de la santé en salle d’opération. Certaines directives régionales et nationales recommandent le recours à des précautions contre la transmission par voie aérienne durant les interventions générant des aérosols pratiquées sur des patients asymptomatiques pendant la pandémie de coronavirus, bien que les données probantes appuyant cette pratique soient limitées.
Conclusion
La transmission virale des patients asymptomatiques en salle d’opération est plausible et les efforts visant à réduire le risque pour les travailleurs de la santé comprennent la réduction de l’aérosolisation et le port d’équipements de protection individuelle adaptés, deux mesures dont la faisabilité variera en fonction du risque géographique et de la disponibilité des équipements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There have been over 5,600,000 confirmed cases of coronavirus disease (COVID-19) and 355,000 deaths worldwide to date (27 May 2020).1 There is concern that viral transmission may occur from patients exhibiting no symptoms, which might pose a risk to healthcare workers (HCWs). In this review, we summarize the available evidence on the risk of asymptomatic spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to HCWs, particularly in locations such as the operating room where aerosol-generating procedures (AGPs) are routinely performed.
Historical context
Severe acute respiratory syndrome coronavirus 2 is not the first pathogenic coronavirus to jump from its animal host to humans. Of the seven known human coronaviruses, four cause mild symptoms similar to the common cold and three others can cause severe illness in humans.2 Severe acute respiratory syndrome (caused by SARS-CoV), Middle East respiratory syndrome (caused by MERS CoV) and COVID-19 (caused by SARS-CoV-2) all result from highly transmissible viruses whose natural reservoir is in bats. Several factors are associated with pathogen spillover from animal hosts to humans and establishment of sustained spread in humans, such as changes in human behaviour, new technologies and industry, changes in land use, increased international travel, microbial adaptation, inadequate public health measures, and sharing our environment with domestic or wild animals.3,4 Given that these factors are unlikely to change, we can expect to see more outbreaks of emerging infectious diseases in the future.5
SARS-CoV-2 mechanism and timing of infection
Respiratory viruses, like SARS-Cov-2, are known to spread by direct contact, such as touching an infected person or a surface infected with virus-containing droplets expelled by an infected person.6 Current recommendations to prevent the spread of SARS-CoV-2, such as frequent hand washing and keeping at least a 2-m distance, would be effective with droplet-borne transmission. Recommendations to practice good hand hygiene, physical distancing, and isolation of infected patients to prevent transmission are common across respiratory viruses and organizational guidelines. There has been controversy, even before the emergence of SARS-CoV-2, over whether respiratory viruses, including coronaviruses (e.g., MERS), can effectively spread via the airborne route and whether contact and droplet precautions are sufficient to prevent transmission.7 The World Health Organization (WHO) recommends droplet and contact precautions for avian influenza and MERS, while the Centers for Disease Control and Prevention takes a more conservative approach, recommending airborne precuations.7 The WHO does recommend additional airborne precautions for novel acute respiratory viruses until transmission patterns can be observed. There is some evidence that SARS-CoV-2 can spread via aerosols, with virus particles found at distances > 2 m from the source for up to three hours.8,9,10 The infectivity of these viral particles, however, remains to be determined.
The median incubation period for SARS-CoV-2 has been estimated to be 5.1 days, with 97.5% of patients developing symptoms within 11.5 days, which is similar to SARS-CoV.11 The upper end of this incubation period forms the rationale for public health recommendations to self-isolate for 14-days after exposure. Several studies have shown that viral loads are highest shortly after symptom onset, gradually decreasing over the ensuing 21 days.12,13,14 Longer periods of viral shedding (up to 30 days) have also been reported.15
Evidence of asymptomatic/pre-symptomatic carriers
The prevalence of asymptomatic (never develops symptoms) or pre-symptomatic (tests positive prior to symptom development) illness has been documented in several populations. Among 565 Japanese nationals evacuated from Wuhan, China and tested for SARS-CoV-2 by real time reverse transcriptase polymerase chain reaction (RT-PCR), 13 tested positive with four (30.8%; 95% confidence interval [CI], 7.7 to 53.8) asymptomatic at the time of testing.16 Of the 3,063 passengers on board the quarantined Diamond Princess Cruise ship who were tested for SARS-CoV-2, 17.9% of those who tested positive were asymptomatic (95% CI, 15.5 to 20.2).17 Residents of a long-term skilled nursing facility with an early outbreak of COVID-19 were all screened for symptoms of illness and viral RNA. Of the 76/82 (93%) residents tested, 23 tested positive of which 13 (57%) were categorized as asymptomatic at the time of testing.18 Seven days later, ten out 13 previously asymptomatic residents developed symptoms and were reclassified as pre-symptomatic during testing. Twenty-four asymptomatic patients in Nanjing, China were characterized after testing positive through screening of close contacts. During hospitalized follow-up, five (20.8%) developed symptoms, 17 (70.8%) showed abnormal computed tomography (CT) scans, and seven (29.2%) had normal CT scans and did not develop symptoms.19 Both targeted testing for SARS-CoV-2 in high-risk Icelandic residents and general Icelandic population screening for SARS-CoV-2 showed asymptomatic patients that tested positive for SARS-CoV-2. In the 1,924 targeted-testing group, 7% had no symptoms, while in the population screening group, 43% of 10,797 Icelandic residents testing positive for the virus reported having no symptoms at the time of testing.20
The available evidence across a range of populations indicates that asymptomatic and pre-symptomatic patients can test positive for SARS-CoV-2 at rates ranging from 17.9% to 57% of those who test positive showing no symptoms. Nevertheless, the overall prevalence of asymptomatic carriers will depend on the distribution and how widespread the disease is in a given population. For example, in the Icelandic population, where less than 1% of the population was positive for SARS-CoV-2 (and 43% of them reporting no symptoms) at the time of screening, the point prevalence of asymptomatic cases was 0.34%.20 In contrast, in a highly infected environment like the Diamond Princess cruise ship where 20% of passengers eventually tested positive, 10% of all passengers and crew were asymptomatic at the time of testing.17
In a study of SARS-CoV-2 upper respiratory viral loads in 18 patients, one asymptomatic patient was included because of close contact with an infected patient. The viral load that was detected in the asymptomatic patient was similar to that in the symptomatic patients, which suggests the transmission potential of asymptomatic or minimally symptomatic patients.21
Cases of asymptomatic transmission
While symptomatic disease is frequently associated with infectivity, there is speculation that for SARS-CoV-2, the latent period (time from exposure to onset of infectiousness) may be shorter than the incubation period (time from exposure to onset of symptoms), leaving a window of time when the patient is infectious but not yet exhibiting symptoms. This is supported by a study of viral loads in Chinese patients, which indicated that pre-symptomatic viral transmission likely occurred.13 Using data from infector-infectee pairs, the authors estimated that viral transmission may have occurred two to three days prior to symptom onset in up to 44% of patients, indicating a transmission pattern more similar to seasonal influenza than SARS-CoV. Of the 157 locally acquired infections identified in Singapore, ten secondary cases (6.4%) were likely acquired prior to the development of symptoms in the index cases. Infections happened on average one to three days before symptom onset in the index cases.22 A familial cluster was identified in Anyang, China where one asymptomatic carrier never developed symptoms but tested positive for the virus, and likely infected five family members.23 In a point-prevalence survey of a skilled nursing facility in King County, Washington, 27 out of 48 (56%) with positive tests were asymptomatic at the time of testing. Given an estimated doubling time of 3.4 days (95% CI, 2.5 to 5.3) in that facility, viral shedding from asymptomatic or pre-symptomatic residents likely contributed to early transmission to other residents and staff members.24
Infection of HCWs
Healthcare workers are at increased risk for infection through occupational exposure to pathogens such as bacteria, fungi, viruses, and parasites.25 Healthcare providers have been affected by SARS-CoV-2, with high reported rates of infection26 and death27 in the UK, although it is unclear how much nosocomial transmission occurred since frontline staff tested positive at similar rates to non-clinical staff and deaths were not reported in ICU HCWs, possibly indicating that current infection control practices in the UK have been effective in limiting occupationally acquired HCW infection. Nevertheless, HCWs have been found to be at higher risk for infection with respiratory pathogens in other settings, particularly those who perform AGPs.28,29 A systematic review showed that, compared with healthcare workers who did not perform aerosol-generating procedures, those who performed tracheal intubation had an increased risk of contracting SARS-CoV in the 2003 epidemic (odds ratio, 6.6) than those who performed non-invasive ventilation (odds ratio, 3.1), tracheotomy (odds ratio, 4.2), and manual ventilation before intubation (odds ratio, 2.8).29 Nevertheless, the authors note very low quality in all included studies, study designs subject to recall bias, and few clear reports of personal protective equipment (PPE) compliance. Low numbers of exposed HCWs also limit the generalizability of the study—in the meta-analysis for risk of HCW infection after tracheal intubation, two of the four included studies had five or fewer HCWs in the exposed group. A separate study found that the protection guidelines failed to thoroughly prevent the transmission of SARS-CoV to HCWs in 2003 and that 9% of the interviewed healthcare workers who had intubated patients contracted SARS. Nevertheless, the cause-effect relationship between infection and intubation in these healthcare workers who contracted SARS was unknown.30
Techniques to minimize risk to HCWs in the operating room
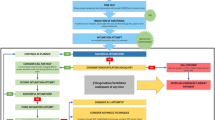
Since asymptomatic transmission is possible with SARS-CoV-2, and HCWs are at risk of infection, techniques to minimize risk in the operating room are needed. Current tests for SARS-CoV-2 are not sensitive enough to eliminate the possibility of asymptomatic carriers entering the operating room. Therefore, other strategies, such as risk reduction through the hierarchy of controls model31 can be employed (see Figure 1). Strategies include delaying elective surgery in patients at high risk for infection, substituting regional anesthesia for AGPs when appropriate, reducing aerosolization, clearing aerosols, and providing appropriate PPE for HCWs.
Examples of controlling exposure to COVID-19 in the operating room using the Centers for Disease Control and Prevention Hierarchy of Controls model31 to implement feasible and effective control strategies to protect healthcare workers from occupational risk. Control methods at the top are potentially more effective and protective.
Preoperative testing
The American Society of Anesthesiologists (ASA) and Anesthesia Patient Safety Foundation jointly recommend preoperative testing of all elective surgery patients where there is SARS-CoV-2 in the region.32 Patients testing positive should be delayed for non-urgent surgery. They caution, however, that the low sensitivity of viral PCR tests means that up to 30% of patients may falsely test negative. Therefore, regardless of a negative test, additional risk-reduction strategies are indicated, including airborne PPE when an AGP is planned.
Reducing aerosolization
Aerosol-generating procedures, such as intubation and mask ventilation, are thought to create small airborne particles that can be suspended in air and travel long distances.33 Under the assumption that asymptomatic and pre-symptomatic patients carrying SARS-CoV-2 have the ability to generate aerosolized virus particles capable of transmission to HCWs during AGPs, there may be ways to reduce the risk of aerosolization. These techniques include avoiding mask ventilation if possible, reducing oxygen flows, having a good seal with the face mask through use of a thenar eminence (“VE”) grip, and ensuring profound paralysis prior to airway instrumentation.34 Aerosolization risk from coughing during extubation can be minimized with lidocaine, dexmedetomidine, remifentanil, and fentanyl.35
Innovative barrier techniques, such as plastic cube boxes, screens, and tents, to reduce aerosolization during airway management of patients with COVID-19 have been described, but further research is required to prove efficacy or recommend routine use for asymptomatic patients, particularly if they hinder timely airway management, as has recently been shown in a simulation study.36,37,38
Operating room ventilation and clearance of aerosols
Severe acute respiratory syndrome and MERS investigations found airborne disease transmission through inefficient hospital ward ventilation systems.39 Fortunately, most operating rooms, owing to their design in protection of the surgical site, have air exchange rates that adequately minimize deposition and floating time of respiratory virus particles (at least 9 per hour).40 Operating room workers should know their local air exchange rates to determine when aerosolized particles are likely to have been cleared, thus reducing the risk of viral transmission to new members of the operating room team entering the room after an AGP has been performed.41
Recommended PPE
While PPE is important, it is only one of the protective measures used to protect HCWs managing airways.42 Early in an epidemic, before viral transmission routes have been well characterized, PPE recommendations for HCWs can be chaotic and evolving.43 During the 2003 SARS epidemic, most virus transmission to HCWs occurred before PPE was routine, or was associated with PPE breaches. Once the epidemic subsided, the dangers of excessive PPE recommendations (e.g., increased contamination during doffing) were recognized, as well as several instances where droplet precautions were effective in preventing transmission, even in settings where intubations occurred.43 Since transmission of SARS-CoV-2 from asymptomatic patients to HCWs has not been reported, there is no clear evidence-based recommendation for appropriate PPE in this setting. Evidence that AGPs actually increase airborne HCW transmission is limited44 and it is uncertain whether N95 masks offer better protection over surgical masks.45,46 Regardless of choice of PPE, adequate training in donning and doffing is essential for ensuring both effectiveness and reducing the risk of self-contamination.47
The ASA recommends the use of airborne PPE for all airway-related AGPs because asymptomatic patients may be harbouring SARS-CoV-2.48 In contrast, the United Kingdom Association of Anaesthetists does not specifically recommend N95 masks for asymptomatic patients, although they acknowledge that as population disease burden increases, it might become necessary to treat all patients as high-risk because of the threat of asymptomatic viral transmission.49,50
Conclusions
There is evidence of asymptomatic and pre-symptomatic carriers of the SARS-CoV-2. While transmission from asymptomatic patients to close contacts has been reported, there have been no reports of asymptomatic patients infecting HCWs during AGPs. The risk that an asymptomatic patient poses to operating room HCWs during AGP will depend on local disease burden.
As health systems begin to consider resuming elective surgery, safely navigating AGPs in those who are asymptomatic will be imperative. Identifying asymptomatic patients who may be infectious using imperfect tests, and maintaining operating room safety for patients and providers, all while avoiding PPE shortages, are some of the challenges faced by health systems around the world. There is still much we do not understand about asymptomatic viral transmission and until more is known, a precautionary approach may expose clinicians to lower risk of infection. Further research on the risk of asymptomatic spread of SARS-CoV-2 and appropriate measures to mitigate this risk, particularly during AGPs, is urgently needed.
References
Johns Hopkins University & Medicine Coronavirus Resource Center. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available from URL: https://coronavirus.jhu.edu/map.html (accessed May 2020).
Ye Z, Yuan S, Yuen K, et al. Zoonotic origins of human coronaviruses. Int J Biol Sci 2020; 16: 1686-97.
Lindahl JF, Grace D. The consequences of human actions on risks for infectious diseases: a review. Infect Ecol Epidemiol 2015. DOI: https://doi.org/10.3402/iee.v5.30048.
Lloyd-Smith J, George D, Pepin KM, et al. Epidemic dynamics at the human-animal interface. Science 2009. DOI: https://doi.org/10.1126/science.1177345.
World Health Organization. Infection prevention and control of epidemic-and pandemic prone acute respiratory infections in health care. WHO guidelines. Available from URL: https://www.who.int/csr/bioriskreduction/infection_control/publication/en/ (accessed May 2020).
La Rosa G, Fratini M, Della Libera S, Iaconelli M, Muscillo M. Viral infections acquired indoors through airborne, droplet or contact transmission. Ann Ist Super Sanita 2013; 49: 124-32.
Shiu EY, Leung NH, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis 2019; 32: 372-9.
van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020; 382: 1564-7.
Santarpia JL, Rivera DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv 2020; DOI: 10.1101/2020.03.23.20039446.
Chen Y, Huang L, Chan C, et al. SARS in hospital emergency room. Emerg Infect Dis 2004; 10: 782-8.
Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020; DOI: https://doi.org/10.7326/M20-0504.
To KK, Tsang OT, Leung W, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020. DOI: https://doi.org/10.1016/S1473-3099(20)30196-1.
He X, Lau EH, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020. DOI: https://doi.org/10.1038/s41591-020-0869-5.
Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis 2020. DOI: https://doi.org/10.1093/cid/ciaa345.
Song R, Han B, Song M, et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing. China. J Infect 2020. DOI: https://doi.org/10.1016/j.jinf.2020.04.018.
Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis 2020. DOI: https://doi.org/10.1016/j.ijid.2020.03.020.
Mizumoto K, Kagaya K, Zarebski A, et al. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 2020; DOI: https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180.
Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 377-81.
Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing. China. Sci China Life Sci 2020. DOI: https://doi.org/10.1007/s11427-020-1661-4.
Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 2020. DOI: https://doi.org/10.1056/NEJMoa2006100.
Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382: 1177-9.
Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 411-5.
Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020. DOI: https://doi.org/10.1001/jama.2020.2565.
Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020. DOI: https://doi.org/10.1056/NEJMoa2008457.
Centers for Disease Control and Prevention. Healthcare Workers, Infectious Agents - NIOSH workplace safety and health topics. Available from URL: https://www.cdc.gov/niosh/topics/healthcare/infectious.html (accessed May 2020).
Hunter E, Price DA, Murphy E, et al. First experience of COVID-19 screening of health-care workers in England. Lancet 2020. DOI: https://doi.org/10.1016/S0140-6736(20)30970-3.
Cook T, Kursumovic E, Lennane S. Exclusive: deaths of NHS staff from covid-19 analysed. Available from URL: https://www.hsj.co.uk/exclusive-deaths-of-nhs-staff-from-covid-19-analysed/7027471.article (accessed May 2020).
Macintyre CR, Seale H, Yang P, et al. Quantifying the risk of respiratory infection in healthcare workers performing high-risk procedures. Epidemiol Infect 2014; 142: 1802-8.
Tran K, Cimon K, Severn M, Pessoa-Silva C, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One 2012. DOI: https://doi.org/10.1371/journal.pone.0035797.
Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA. Intubation of SARS patients: infection and perspectives of healthcare workers. Can J Anesth 2006; 53: 122-9.
Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health, (NIOSH). Hierarchy of Controls. Available from URL: https://www.cdc.gov/niosh/topics/hierarchy/default.html (accessed May 2020).
American Society of Anesthesiologists. The ASA and APSF Joint Statement on Perioperative Testing for the COVID-19 Virus. Available from URL: https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/asa-and-apsf-joint-statement-on-perioperative-testing-for-the-covid-19-virus (accessed May 2020).
Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect 2006; 64: 100-14.
Brewster DJ, Chrimes N, Do TB, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust 2020. DOI: https://doi.org/10.5694/mja2.50598.
Tung A, Fergusson NA, Ng N, Hu V, Dormuth C, Griesdale DG. Pharmacological methods for reducing coughing on emergence from elective surgery after general anesthesia with endotracheal intubation: protocol for a systematic review of common medications and network meta-analysis. Syst Rev 2019. DOI: https://doi.org/10.1186/s13643-019-0947-2.
Au Yong PS, Chen X. Reducing droplet spread during airway manipulation: lessons from the COVID-19 pandemic in Singapore. Br J Anaesth 2020. DOI: https://doi.org/10.1016/j.bja.2020.04.007.
Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier enclosure during endotracheal intubation. N Engl J Med 2020. DOI: https://doi.org/10.1056/NEJMc2007589.
Begley JL, Lavery KE, Nickson CP, Brewster DJ. The aerosol box for intubation in COVID-19 patients: an in-situ simulation crossover study. Anaesthesia 2020. DOI: https://doi.org/10.1111/anae.15115.
Yu HC, Mui KW, Wong LT, Chu HS. Ventilation of general hospital wards for mitigating infection risks of three kinds of viruses including Middle East respiratory syndrome coronavirus. Indoor Built Environ 2017. DOI: https://doi.org/10.1177/1420326X16631596.
Memarzadeh F, Manning AP. Comparison of operating room ventilation systems in the protection of the surgical site. ASHRAE Trans 2002; 108: 3-15.
Centers for Disease Control and Prevention. Guidelines for Environmental Infection Control in Health-Care Facilities (2003) Appendix B: Air. Available from URL: https://www.cdc.gov/infectioncontrol/guidelines/environmental/appendix/air.html (accessed May 2020).
Weissman DN, de Perio MA, Radonovich,Lewis J Jr. COVID-19 and risks posed to personnel during endotracheal intubation. JAMA 2020; DOI: 10.1001/jama.2020.6627.
Nicolle L. SARS safety and science. Can J Anesth 2003. DOI: https://doi.org/10.1007/BF03018360.
Wilson NM, Norton A, Young FP, Collins DW. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia 2020. DOI: https://doi.org/10.1111/anae.15093.
Smith JD, MacDougall CC, Johnstone J, Copes RA, Schwartz B, Garber GE. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ 2016; 188: 567-74.
Bartoszko JJ, Farooqi MA, Alhazzani W, Loeb M. Medical masks vs N95 respirators for preventing COVID-19 in healthcare workers: a systematic review and meta-analysis of randomized trials. Influenza Other Respir Viruses 2020. DOI: https://doi.org/10.1111/irv.12745.
Roberts V. To PAPR or not to PAPR? Can J Respir Ther 2014; 50: 87-90.
American Society of Anesthesiologists. The Use of Personal Protective Equipment by Anesthesia Professionals during the COVID-19 Pandemic. Available from URL: https://www.asahq.org/about-asa/newsroom/news-releases/2020/03/update-the-use-of-personal-protective-equipment-by-anesthesia-professionals-during-the-covid-19-pandemic (accessed May 2020).
Cook TM, El-Boghdadly K, McGuire B, et al. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020. DOI: https://doi.org/10.1111/anae.15054.
Association of Anaesthetists. Anaesthetic Management of Patients During a COVID-19 Outbreak. Available from URL: https://anaesthetists.org/Home/Resources-publications/Anaesthetic-Management-of-Patients-During-a-COVID-19-Outbreak (accessed May 2020).
Acknowledgements
The authors would like to express their appreciation for support from the Royal Columbian Hospital Department of Anesthesia and the Royal Columbian Hospital Foundation Anesthesia Innovation Fund.
Author information
Authors and Affiliations
Contributions
Susan M. Lee, Paula Meyler, and Michelle Mozel contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Tonia Tauh contributed to the conception and design of the study. Richard Merchant contributed to the interpretation of data.
Corresponding author
Ethics declarations
Disclosures
None.
Funding statement
This work was supported by the Royal Columbian Hospital Foundation Anesthesia Innovation Fund.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, S., Meyler, P., Mozel, M. et al. Asymptomatic carriage and transmission of SARS-CoV-2: What do we know?. Can J Anesth/J Can Anesth 67, 1424–1430 (2020). https://doi.org/10.1007/s12630-020-01729-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01729-x