Abstract
Purpose
Intensive care physicians play an important role in the identification and referral of potential organ donors in Canada. Nevertheless, little is known about intensivists’ attitudes or behaviours in situations where families override previously expressed consent to donate; nor why physicians elect not to refer patients who are potential donors to provincial organ donation organizations (physician non-referral).
Methods
We integrated questions regarding family override and physician non-referral into an online, self-administered survey of Canadian intensivists. We report results descriptively.
Results
Fifty percent of targeted respondents (n = 550) participated. Fifty-five percent reported having witnessed family override situations and 44% reported having personally not referred patients who were potential donors. Fifty-six percent of respondents stated they would not pursue donation in the face of family override; 2% stated they would continue with the donation process. Fear of loss of trust in the donation system (81%) and obligation to respect the grief and desires of surrogate decision makers (71%) were frequently reported reasons to respect family override requests. Respondents who chose not to refer patients often did so based on organ dysfunction they assumed would preclude donation (59%), or a perception that the family was too distressed to consider donation (42%). No respondents reported that personally held beliefs against organ donation influenced their decision.
Conclusion
Physicians caring for patients who are potential organ donors commonly encounter both family override and physician non-referral situations. Knowledge translation of optimal practices in identification and referral could help ensure that physician practices align with legal requirements and practice recommendations.
Résumé
Objectif
Les intensivistes jouent un rôle important dans l’identification et la référence des donneurs potentiels d’organes au Canada. Toutefois, nous ne connaissons que très peu de choses concernant les attitudes et comportements des intensivistes dans les situations dans lesquelles les familles vont à l’encontre d’un consentement de don exprimé au préalable; nous ne savons pas non plus pourquoi certains médecins décident de ne pas référer des patients qui seraient de potentiels donneurs aux organismes de dons d’organes provinciaux (non-référence médicale).
Méthode
Nous avons intégré des questions concernant la décision de la famille de ne pas respecter une décision de don d’organes et la non-référence médicale dans un sondage auto-administré en ligne envoyé aux intensivistes canadiens. Nous rapportons les résultats du sondage de façon descriptive.
Résultats
Cinquante pourcent des répondants ciblés (n = 550) ont participé. Cinquante-cinq pourcent ont rapporté avoir été témoins de situations dans lesquelles la décision de la famille allait à l’encontre des souhaits de la personne décédée et 44 % ont rapporté avoir personnellement décidé de ne pas référer certains patients alors qu’ils étaient des donneurs potentiels. Cinquante-six pourcent des répondants ont déclaré qu’ils ne chercheraient pas à encourager un don d’organes si la famille y était opposée; 2 % ont déclaré qu’ils poursuivraient le processus de don. La peur d’une perte de confiance dans le système de don (81 %) et l’obligation de respecter le deuil et les souhaits des mandataires (71 %) comptaient parmi les raisons fréquemment citées de respecter les demandes de la famille plutôt que celles de la personne décédée. Les répondants ayant choisi de ne pas référer leurs patients ont souvent pris cette décision en raison d’une atteinte des organes qui, selon eux, aurait exclu le don (59 %), ou d’une perception selon laquelle la famille était trop bouleversée pour envisager le don (42 %). Aucun répondant n’a rapporté que ses convictions personnelles contre le don d’organes auraient influencé sa décision.
Conclusion
Les médecins qui s’occupent de patients qui sont des donneurs potentiels se retrouvent souvent dans des situations où la volonté de la famille l’emporte ou dans des situations de non-référence médicale. La transmission des connaissances concernant les meilleures pratiques dans l’identification et la référence des patients pourrait aider à garantir que les pratiques médicales soient en accord avec les exigences légales et les recommandations de pratique.
Similar content being viewed by others
In the last ten years, Canada has seen a sustained increase in organ donation, with a rise from 14.1 to 20.9 donors per million population.1 While encouraging, death and disability of transplant waiting list patients is an ongoing challenge. Over 80% of transplants arise from deceased organ donors and are predicated on consent. Actual consent rates are steady at approximately 60%,2,3,4 while support for donation among the general public and healthcare professionals is nearly 90%,Footnote 1,5 suggesting that consent practices could be improved.
The majority of patients who are potential organ donors are identified in the intensive care unit (ICU), and most have suffered severe neurologic injury. Consent must be obtained from their family or surrogate decision makers (SDMs). While local practice may vary, the attending ICU physician is usually the healthcare worker primarily responsible for referring patients who are potential donors to organ donation organizations (ODOs) and is sometimes involved in discussing consent. Despite their key role in the donation process, little is known about the knowledge, attitudes, or behaviours of Canadian ICU physicians towards identification and referral of potential donors or consent conversations with SDMs. The most recent survey to explore physician opinions on donation consent issues was done in 2006.5
Definitions
Consistent with other recent Canadian publications,6 family override was defined as SDM refusal of consent to proceed with donation despite documentation of previous desire to donate by the patient who is a potential donor (e.g., in a donation registry). The case from the survey described in the Figure details a family override situation.
Physician non-referral was defined as a physician choice not to refer a patient who is a potential donor and/or discouragement of contact between the treating team and the ODO. In this situation, the desires of the patient and the SDMs to pursue donation are unknown.
Objective
The objective of this survey was to investigate adult and pediatric intensive care physicians’ knowledge, attitudes, and reported behaviour in situations of potential family override or physician non-referral to consent for deceased organ donation.
Methods
We conducted a cross-sectional self-administered survey of Canadian intensive care physicians. Questions specifically investigating family override or physician non-referral situations were embedded in a larger survey on deceased donation practices in Canada. We followed a standardized approach for the design and conduct of self-administered surveys.7 The survey was a combination of stand-alone questions and case-based scenarios. Questions were multiple choice with options of either yes/no/unsure or five-point Likert scales when appropriate. The survey questions pertaining to this manuscript can be accessed as Electronic Supplementary Material (eAppendix 1). This survey was approved by the research ethics committee of the Centre hospitalier de l’Université de Montréal.
Population
Our sampling frame included intensivists practicing in Canadian institutions that may care for potential organ donors. Intensivists were identified from Canadian Blood Services and the membership list of the Canadian Critical Care Society, supplemented by manual searches of publicly available sources (e.g., hospital websites). Eligible respondents were physicians in independent practice who reported the potential to be involved with the deceased organ donation process either as the most responsible attending physician or a consultant. Physicians-in-training (residents, fellows) were excluded.
Survey development
A steering committee that included experts in critical care, neurocritical care, epidemiology, and survey methods, law, organ donation, organ transplantation, and social science, as well as a patient representative formed the expert panel. The process closely followed recommendations for the development of self-administered surveys,7 including item generation, item reduction, survey creation, dissemination of the survey, analysis, and interpretation of the results. This process encompassed the development of the entire survey, not just the sections reported here.
In this first phase of survey development, the steering committee identified the pertinent domains of eligibility evaluation for deceased organ donation, the use of ancillary tests, the consent process, and clinicians’ attitudes towards organ donation. Using the online platform Lime Survey™, the expert panel reviewed 74 potential items relevant to the domains reported in this manuscript.
The second phase of survey development used a Delphi approach among the steering committee to rank items within each domain. Items were ranked on a five-point Likert scale and 30 items that received a score ≥ 4 by 75% of respondents were retained. We (M.W. and M.C.) edited these items, added sub-items for ease of response, and regrouped questions by domain. This resulted in the 26 items included in eAppendix 2 (available as Electronic Supplementary Material [ESM]). Questions in the original domains not reported in this manuscript (e.g., items ancillary testing for neurologic death determination) will be reported in subsequent manuscripts.
The final survey included a mix of questions allowing selection from responses (with a free text option) and closed answers (binary and five-level Likert scales) (eAppendix 1 as ESM). The survey included both independent questions and questions referring to clinical scenarios to help assess if and why respondent opinions changed according to different clinical situations. For closed questions, we used an “other” option where appropriate to avoid a floor and ceiling effect.7 The survey also included a description of respondent characteristics.
Survey validation
This initial version of the survey was evaluated for face validity by all steering committee members. The survey was then pilot-tested among trainees (six residents and fellows) who were representative of our target population but ineligible for the definitive survey. At this stage, we studied the flow, acceptability, ease of administration, time required, redundancy, and comprehensiveness of the questions. After edits based on pilot testing feedback, the clinical scenarios of the survey were again assessed for comprehensiveness, clarity, and face validity by members of the steering committee (M.W. and M.C.). Reliability and content validity were assessed through test-retest reliability with the same trainees responding to the survey two weeks after first exposure. We did not study interrater reliability and internal consistency because we did not have an a priori expected pattern of responses among respondents (interrater reliability), and factor analysis calculations (for internal consistency) would have required approximately 100 respondents from our already limited respondent pool.
Survey administration
The survey was disseminated using an online platform (LimeSurvey™). Respondents were contacted by email. To ensure that respondents met the inclusion criteria, a screening section confirmed eligibility and consent for the study. Each survey was identified by a unique online identifier and answers were collected anonymously. The identifier was linked to an email address and each respondent was allowed to complete the survey only once. Two reminders were sent by email to non-responders at 14-day intervals. Respondents received no compensation for their participation in the survey.
Data analysis
All survey responses are reported as descriptive statistics (percentages). The denominator of respondents has been adjusted for each question to remove those who did not respond to that question. For reporting of Likert scale items, respondents who responded “agree” or “strongly agree” were grouped together.
The proportion of respondents who had a self-identified role as a donation physician was high, but as there was no planned analysis of this subgroup, we did not generate comparative statistics of their responses. We have reported some of the donation physicians’ responses descriptively in Tables 2 and 3 below. Free text responses were not analyzed for this report.
Sample size
The exact number of physicians who could potentially identify and refer a deceased donor in Canada is not known. For the purposes of this study, we focused only on intensive care physicians. Based on recent Canadian surveys, we a priori estimated between 300 and 400 intensivists would be eligible for this study.8,9,10 For a confidence level of 95% and a 5% margin of error, between 169 and 196 respondents were a priori required to obtain clinically meaningful results, corresponding to a response rate of 49–56%, which is in-line with previous work in a similar population.8
Results
Respondents
We approached 550 potential respondents between February 26, 2018 to March 26, 2018; 21 did not meet inclusion criteria and one declined participation. The response rate was 50% (263/529) and 89% (235/263) of respondents fully completed the survey, yielding a margin of error of 4–5% for the questions with the lowest and highest response rates respectively at a confidence level of 95%.
The demographics of the participants are detailed in Table 1. The majority of respondents were associated with academic institutions (92%, 239/261) and 13% (35/263) worked in pediatric ICUs. Twenty-two percent (58/263) of respondents reported that they had a defined role as a donation specialist in their hospital or institution.
General attitudes towards donation
Overall, 95% of respondents had a mostly positive (23%, 55/235) or positive (72%, 170/235) general opinion regarding organ donation. No respondent had a negative opinion of donation, 1% (3/235) had a mostly negative opinion, and 3% (7/235) had a neutral opinion.
Family override
When questioned regarding the recalled frequency of family override events, 55% (128/231) stated they had personally witnessed such an occurrence at some point in their career. Most (91%, 116/128) had seen one to five events with 3% (4/128) having seen more than ten events.
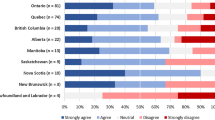
About half (56%, 131/234) of respondents stated they would respect the SDM’s decision and pursue withdrawal of life sustaining therapy without donation despite a previously expressed intent to donate as described in the scenario in the Figure. In follow-up questions, respondents were asked to select reasons why they would follow a family override request (Table 2). Five (2%, 5/234) said they would continue with donor management and donation procedures despite the objection of the SDM. A minority of respondents stated that they would seek further guidance in the form of a legal opinion (8%, 18/234) or an ethics consultation (16%, 38/234). Among those requesting an ethics consult, the majority (61%, 23/38) stated they would not proceed with donation against SDM wishes, even if that was the conclusion of the ethics committee. Ten percent (27/263) expressed that the law in their jurisdiction supports pursuing donation despite SDM objection.
For most respondents (80%, 188/235) the patient being a potential donor after circulatory determination of death as opposed to after neurologic determination of death did not affect their likelihood to respect a family override request.
Physician non-referral
Forty-four percent of respondents (103/239) stated that they personally had not referred patients who were potential donors at some point in their career. Table 3 shows the responses selected by physicians to explain why they had not referred patients. No respondent stated that they did not refer because of personally held beliefs against donation or transplantation.
Respondents who reported they had never personally chosen not to refer patients were asked if they had observed colleagues not refer patients who were potential donors and 43% (58/135) stated that they had seen this. Perceived reasons for the lack of referral were broadly similar as for respondents’ personal reasons for a lack of referral (data not shown). The exception was that while 33% (19/58) of respondents believed their colleagues did not refer because of the colleague’s personally held beliefs against donation and transplantation, no respondent stated that was a reason they personally had not referred.
Discussion
Our survey revealed substantial variation among Canadian physicians regarding beliefs, attitudes, and behaviours in how to manage challenging deceased donation consent situations. Family override scenarios, while uncommon, were familiar to respondents, and more than half of them would respect SDMs’ desires to halt donation proceedings with no further legal or ethics consultation. Physician non-referral was a frequently reported behaviour, either by participants themselves or observed among their colleagues. The degree of general support for deceased donation was high among respondents, consistent with past surveys.5
A clinician faced with a family override request is in an ethically complex situation. They must balance the obligation to respect the previously expressed intent of the patient against the currently expressed intent of surviving SDMs. While these situations are emotionally charged and ethically ambiguous, Canadian legislation is largely clear on this issue. In a 2016 analysis of donation legislation the authors found that in almost all provinces previously expressed intent for donation is legally binding and should be overturned only in exceptional circumstances.6 Application of these laws to pursue donation against a family override, however, has not been tested in court, and provincial ODOs often discourage providers from proceeding with donation against expressed override. Few respondents in our survey were aware of this potential legal obligation to pursue donation, and many expressed a concern over possible legal action if they were to pursue donation against family override. Almost none stated they would pursue donation in the face of family override, even though the laws in most provinces state they should respect the autonomy of the deceased. These issues point to a lack of alignment between legislation, physician beliefs, and donation practices by clinicians and ODOs. Our survey was not designed to identify factors that could decrease that lack of alignment, but the results suggest that efforts should be made to increase clinician and ODO understanding of the legal framework that governs a potential family override situation.
There was also a frequently expressed desire to respect family override requests to protect public trust in the donation system. It is unclear, however, if that concern is justified. In a recent analysis of Canadian media, 61% of identified articles argued against respecting family override and 80% identified a need to clarify or eliminate family override practices.11 This is consistent with polling by Canadian Blood Services where 82% of the general public stated that the desires of the deceased should take precedence over the desires of surviving SDMs.A A better understanding of the impact of family override on public trust would require a broad public debate based on an open analysis of public opinion, existing legislation, and current donation practices.
The impact of physician non-referral on the number of actual organ donors and organs transplanted is difficult to estimate, because the nationwide incidence is unknown and the consent rate of SDMs who were not referred is unknown. Independent of the impact on donation system performance measures, there are reasons to call into question the practice of physician non-referral. Physician non-referral moves the locus of control away from the patient and SDMs and towards the medical team. By not offering the possibility to explore donation, SDMs are not able to autonomously express their or the patient’s desire to donate. In addition, we observed that many of the reasons to not refer involved apparent misunderstandings of recommendations and practices, such as the perception that the patient had organ dysfunction that would preclude donation. While ODOs do exclude patients with organ dysfunction, the specific levels of dysfunction that exclude a patient change frequently and are context dependent. ODOs generally encourage clinicians to refer a broad spectrum of patients for evaluation.12 Knowledge translation initiatives designed to disseminate these practices may improve referral and ultimately donation rates.13
Similar to family override, legislation exists in several provinces that would seem to limit the practice of physician non-referral. Mandatory referral of patients is currently required by law in six provinces.12 Nevertheless, few hospitals or healthcare systems have processes to actively ensure compliance with this legislation,12 and our results affirm that many clinicians do not refer these patients in a systematic manner. Questions related specifically to mandatory referral were included in the broader survey and will be reported in future manuscripts.
Our findings also suggest that personally held beliefs against donation is a rare cause of physician non-referral. None of the respondents who had personally not referred patients stated this was the reason they had not referred, while roughly one third believed that this motivated their colleagues to not refer. This discrepancy is difficult to explain. Possible explanations include a misreading of their colleagues’ motivations or a selection bias of people with favourable attitudes towards donation to respond to the survey. Also possible is a reluctance to express views that may go against the general acceptance of donation in the ICU community, even in an anonymous survey. It may also be due to a lack of recognition of the impact of unrecognized bias on behaviour, a phenomenon described elsewhere in studies of implicit bias.14 This discrepancy points to an area that deserves further study, including the impact of more closely monitoring and feeding back identification and referral rates. Nevertheless, the high rate of positive attitudes towards donation suggests interventions aimed to decrease physician non-referral by improving attitudes towards donation in general may not be the most effective use of implementation resources.
Strengths and limitations
The survey was rigorously developed, and our sampling frame included a comprehensively validated list of practicing ICU physicians with a reasonable response rate.
There were, however, limitations. Non-respondents possibly had significantly different attitudes or behaviours than those who chose to participate. Related to this point, our survey included a high number of intensivists who had formal roles in the donation system. The descriptive reports included in Tables 2 and 3 do not suggest a substantial difference between these groups, though a more detailed analysis of these and other results will be performed for future reports. A similar analysis of differences between high and low volume centres will be performed, as the donation expertise in these settings is likely variable. Finally, this was a quantitative survey that did not allow respondents to provide nuanced responses regarding these difficult ethical situations.
Follow-up research will be required to assess how to integrate these findings into referral and consent practices. Further study of public and other healthcare worker opinion related to both family and physician non-referral needs to be performed to correlate physician perceptions with actual public opinion.
Conclusion
Experience with family override and physician non-referral situations are reported by almost 50% of surveyed Canadian intensivists. The exact impact of not pursuing donation in these settings is difficult to estimate, but fewer referred potential donors surely results in fewer instances of donation and transplantation. Physicians expressed a misunderstanding of the existing law in regard to family override and frequently chose not to refer patients despite mandatory referral legislation. Broad stakeholder consultation is necessary to increase alignment between physician practices, organ donation organization policies, and organ donation legislation.
Notes
Canadian Blood Services. Spring 2017 Organ and Tissue Donation General Public Survey. Administered by IPSOS on behalf of the Canadian Blood Services. Available upon request.
References
Canadian Blood Services. Organ donation and transplantation in Canada - System Progress Report 2006–2015. 2016: 1-98. Available from URL: https://www.blood.ca/sites/default/files/ODT_Report.pdf (accessed September 2019).
National Health Service, Blood and Transplant. National Potential Donor Audit: April 2016 - March 2017. 2017 Aug 2:1–20. Available from URL: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/4660/section_13_national_potential_audit_donors.pdf (accessed September 2019).
Goldberg DS, Halpern SD, Reese PP. Deceased organ donation consent rates among racial and ethnic minorities and older potential donors. Crit Care Med 2013; 41: 496-505.
Trillium Gift of Life Network. 2017/18 Highlights Report. 2018: 1-6. Available from URL: https://www.giftoflife.on.ca/resources/pdf/TGLN_17-18_Highlights_FinalEN.pdf (accessed September 2019).
Canadian Council for Donation and Transplantation. Health Professional Awareness and Attitudes on Organ and Tissue Donation and Transplantation Including Donation after Cardiocirculatory Death. 2006: 1-84. Available from URL: https://professionaleducation.blood.ca/sites/msi/files/Survey-Health-Prof.pdf (accessed September 2019).
Toews M, Caulfield T. Evaluating the “family veto” of consent for organ donation. CMAJ 2016; 188: E436-7.
Burns KE, Duffett M, Kho ME, et al. A guide for the design and conduct of self-administered surveys of clinicians. CMAJ 2008; 179: 245-52.
Turgeon AF, Lauzier F, Burns KE, et al. Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Crit Care Med 2013; 41: 1086-93.
McIntyre LA, Hébert PC, Fergusson D, Cook DJ, Aziz A; Canadian Critical Care Trials Group. A survey of Canadian intensivists’ resuscitation practices in early septic shock. Crit Care BioMed Central 2007; DOI: 10.1186/cc5962.
Lamontagne F, Cook DJ, Adhikari NK, et al. Vasopressor administration and sepsis: a survey of Canadian intensivists. J Crit Care 2011; 26(532): e1-7.
Anthony SJ, Toews M, Caulfield T, Wright L. Family veto in organ donation in Canada: framing within English-language newspaper articles. CMAJ Open 2017; 5: E768-72.
Zavalkoff S, Shemie SD, Grimshaw JM, et al. Potential organ donor identification and system accountability: expert guidance from a Canadian consensus conference. Can J Anesth 2019; 66: 432-47.
de la Rosa G, Dominguez-Gil B, Matesanz R, et al. Continuously evaluating performance in deceased donation: the Spanish Quality Assurance Program. Am J Transplant 2012; 12: 2507-13.
Maina IW, Belton TD, Ginzberg S, Singh A, Johnson TJ. A decade of studying implicit racial/ethnic bias in healthcare providers using the implicit association test. Soc Sci Med 2018; 199: 219-29.
Author contributions
Matthew J. Weiss and Michaël Chassé contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Shane W. English, Frederick D’Aragon, François Lauzier, Alexis F. Turgeon, Sonny Dhanani, Lauralyn McIntyre, Sam D. Shemie, Gregory Knoll, Dean A. Fergusson, Samantha J. Anthony, David Hartell, and Jim Mohr contributed to the study conception and design. Adnan Haj-Moustafa contributed to the acquisition and analysis of data. Shane W. English, Frederick D’Aragon, François Lauzier, Alexis F. Turgeon, Sonny Dhanani, Lauralyn McIntyre, Sam D. Shemie, Gregory Knoll, Dean A. Fergusson, Samantha J. Anthony, and Adnan Haj-Moustafa contributed to the interpretation of data.
Acknowledgements
We thank all the respondents who offered their time to thoughtfully respond to this survey. Livia Carvalho assisted with preparation of the tables and editing of the final manuscript. We also would like to thank Drs. Kusum Menon and Bram Rochwerg from the Canadian Critical Care Trials Group for offering their insightful reviews of this manuscript prior to submission.
Conflicts of interest
None.
Funding statement
This survey was supported with funds from the Fondation du Centre Hospitalier de l’Université Montréal. The Canadian Critical Care Society and Canadian Blood Services provided in-kind support for development of the contact list. Drs. Lauzier, D’Aragon and Chassé are recipients of a research salary support Award from the Fonds de Recherche du Québec—Santé (FRQS). Dr. Turgeon is the Chairholder of the Canada Research Chair in Critical Care Neurology and Trauma. The Canadian Critical Care Trials Group receives funding from a Canadian Institute of Health Research grant.
Editorial responsibility
This submission was handled by Dr. Sangeeta Mehta, Associate Editor, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2020; 67: this issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12630_2019_1538_MOESM1_ESM.pdf
Supplementary material 1 (PDF 159 kb) eAPPENDIX 1 Survey of Canadian intensivists’ attitudes towards deceased organ donation
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weiss, M.J., English, S.W., D’Aragon, F. et al. Survey of Canadian intensivists on physician non-referral and family override of deceased organ donation. Can J Anesth/J Can Anesth 67, 313–323 (2020). https://doi.org/10.1007/s12630-019-01538-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01538-x