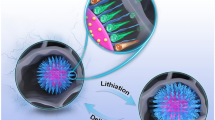
Abstract
To improve the electrochemical kinetics of Nd-Mg-Ni alloy electrodes, the alloy surface was modified with highly conductive reduced graphene oxide (rGO) via a chemical reduction process. Results indicated that rGO sheets uniformly coated on the alloy surface, yielding a three-dimensional network layer. The coated surfaces contained numerous hydrophilic functional groups, leading to better wettability of the alloy in aqueous alkaline media. This, in turn, increased the concentration of electro-active species at the interface between the electrode and the electrolyte, improving the electrochemical kinetics and the rate discharge of the electrodes. The high rate dischargeability at 1500 mA·g−1 increased from 53.2% to 83.9% after modification. In addition, the modification layer remained stable and introduced a dense metal oxide layer to the alloy surface after a long cycling process. Therefore, the protective layer prevented the discharge capacity from quickly decreasing and improved cycling stability.
Similar content being viewed by others
References
J. Zhang, G.J. Shao, W.W. Guo, Y.W. Lou, and B.J. Xia, Estimating the state of charge of MH-Ni batteries by measuring their stable internal pressure, J. Power Sources, 343(2017), p. 183.
W.C.M. de Oliveira, G.D. Rodrigues, A.B. Mageste, and L.R. de Lemos, Green selective recovery of lanthanum from Ni-MH battery leachate using aqueous two-phase systems, Chem. Eng. J., 322(2017), p. 346.
L.A. Benavides, D.J. Cuscueta, H. Troiani, A.A. Ghilarducci, and H.R. Salva, Effect of carbon nanotubes purification in the performance of a negative electrode of a Ni/MH battery, Int. J. Hydrogen Energy, 39(2014), No. 16, p. 8841.
L.Z. Ouyang, J.L. Huang, H. Wang, J.W. Liu, and M. Zhu, Progress of hydrogen storage alloys for Ni-MH re-chargeable power batteries in electric vehicles: A review, Mater. Chem. Phys., 200(2017), p. 164.
G.H. Ağaoğlu and G. Orhan, Production and electrochemical characterization of MgNi alloys by molten salt electrolysis for Ni-MH batteries, Int. J. Hydrogen Energy, 43(2018), No. 12, p. 6266.
Y.F. Liu, Y.H. Cao, L. Huang, M.X. Gao, and H.G. Pan, Rare earth-Mg-Ni-based hydrogen storage alloys as negative electrode materials for Ni/MH batteries, J. Alloys Compd., 509(2011), No. 3, p. 675.
X. Tian, G. Yun, H.Y. Wang, T. Shang, Z.Q. Yao, W. Wei, and X.X. Liang, Preparation and electrochemical properties of La-Mg-Ni-based La0.75Mg0.25Ni3.3Co0.5 multiphase hydrogen storage alloy as negative material of Ni/MH battery, Int. J. Hydrogen Energy, 39(2014), No. 16, p. 8474.
Y.M. Zhao, S.M. Han, Y. Li, J.J. Liu, L. Zhang, S.Q. Yang, J. Cao, and Z.R. Jia, Structural phase transformation and electrochemical features of La-Mg-Ni-based AB4-type alloys, Electrochim. Acta, 215(2016), p. 142.
J.G. Yuan, W. Li, and Y. Wu, Hydrogen storage and low-temperature electrochemical performances of A2B7 type La-Mg-Ni-Co-Al-Mo alloys, Prog. Nat. Sci., 27(2017), No. 2, p. 169.
Y.F. Liu, H.G. Pan, M.X. Gao, and Q.D. Wang, Advanced hydrogen storage alloys for Ni/MH rechargeable batteries, J. Mater. Chem., 21(2011), p. 4743.
S. Ma, M.X. Gao, R. Li, H.G. Pan, and Y.Q. Lei, A study on the structural and electrochemical properties of La0.7-xNdxMg0.3Ni2.45Co0.75Mn0.1Al0.2(x=0.0-3.0) hydrogen storage alloys, J. Alloys Compd., 457(2008), No. 1–2, p. 457.
Y. Li, S.M. Han, J.H. Li, X.L. Zhu, and L. Hu, The effect of Nd content on the electrochemical properties of low-Co La-Mg-Ni-based hydrogen storage alloys, J. Alloys Compd., 458(2008), No. 1–2, p. 357.
Y. Li, Y. Tao, and Q. Huo, Effect of stoichiometry and Cu-substitution on the phase structure and hydrogen storage properties of Ml-Mg-Ni-based alloys, Int. J. Miner. Metall. Mater., 22(2015), No. 1, p. 86.
S.Q. Yang, H.P. Liu, S.M. Han, Y. Li, and W.Z. Shen, Effects of electroless composite plating Ni-Cu-P on the electrochemical properties of La-Mg-Ni-based hydrogen storage alloy, Appl. Surf. Sci., 271(2013), p. 210.
B.P. Wang, L.M. Zhao, C.S. Cai, and S.X. Wang, Effects of surface coating with polyaniline on electrochemical properties of La-Mg-Ni-based electrode alloys, Int. J. Hydrogen Energy, 39(2014), No. 20, p. 10374.
Y. Li, Y. Tao, D.D. Ke, Y.F. Ma, and S.M. Han, Electrochemical kinetic performances of electroplating Co-Ni on La-Mg-Ni-based hydrogen storage alloys, Appl. Surf. Sci., 357(2015), p. 1714.
M. Dymek, M. Nowak, M. Jurczyk, and H. Bala, Encapsulation of La1.5Mg0.5Ni7 nanocrystalline hydrogen storage alloy with Ni coatings and its electrochemical characterization, J. Alloys Compd., 749(2018), p. 534.
L.L. Xiao, Y.J. Wang, Y. Liu, D.W. Song, L.F. Jiao, and H.T. Yuan, Influence of surface treatments on microstructure and electrochemical properties of La0.7Mg0.3Ni2.4Co0.6 hydrogen-storage alloy, Int. J. Hydrogen Energy, 33(2008), No. 14, p. 3925.
A. Matsuyama, H. Mizutani, T. Kozuka, and H. Inoue, Effect of surface treatment with boiling alkaline solution on electrochemical properties of the ZrNi alloy electrode, Int. J. Hydrogen Energy, 41(2016), No. 23, p. 9908.
W.H. Zhou, Z.Y. Tang, D. Zhu, Z.W. Ma, C.L. Wu, L.W. Huang, and Y.G. Chen, Low-temperature and instantaneous high-rate output performance of AB5-type hydrogen storage alloy with duplex surface hot-alkali treatment, J. Alloys Compd., 692(2017), p. 364.
R.C. Cui, C.C. Yang, M.M. Li, B. Jin, X.D. Ding, and Q. Jiang, Enhanced high-rate performance of ball-milled MmNi3.55Co0.75Mn0.4Al0.3 hydrogen storage alloys with graphene nanoplatelets, J. Alloys Compd., 693(2017), p. 126.
L.J. Huang, W. Y.X. Wang, H. Zhen, J.G. Tang, W. Yao, L. J.X. Liu, J. J.Q. Liu, L. J.Q. Liu, and L.A. Belfiore, Effects of graphene/silver nanohybrid additives on electrochemical properties of magnesium-based amorphous alloy, J. Power Sources, 269(2014), p. 716.
L.J. Huang, Y.X. Wang, J.G. Tang, Y. Wang, J.X. Liu, Z. Huang, J.Q. Jiao, W. Wang, M.J. Kipper, and L. A. Belfiroe, A new graphene nanocomposite to improve the electrochemical properties of magnesium-based amorphous alloy, Mater. Lett., 160(2015), p. 104.
N. Li, Y. Du, Q.P. Feng, G.W. Huang, H.M. Xiao, and S.Y. Fu, A novel type of battery-supercapacitor hybrid device with highly switchable dual performances based on a carbon skeleton/Mg2Ni free-standing hydrogen storage electrode, ACS Appl. Mater. Interfaces, 9(2017), No. 51, p. 44828.
Z.Q. Lan, K. Zeng, B. Wei, G.X. Li, H. Ning, and J. Guo, Nickel-graphene nanocomposite with improved electrochemical performance for La0.7Mg0.3(Ni0.85Co0.15)3.5 electrode, Int. J. Hydrogen Energy, 42(2017), No. 17, p. 12458.
J. Zhang, H. Qu, S. Yan, L. R. Yin, and D.W. Zhou, Dehydrogenation properties and mechanisms of MgH2-NiCl2 and MgH2-NiCl2-graphene hydrogen storage composites, Met. Mater. Int., 23(2017), No. 4, p. 831.
Z.Q. Lan, Z.Z. Sun, Y.C. Ding, N. Hua, W.L. Wei, and G. Jin, Catalytic action of Y2O3@graphene nanocomposite on the hydrogen-storage property of Mg-Al alloy, J. Mater. Chem. A, 5(2017), No. 29, p. 15200.
H.J. Wu, Z.Z. Sun, J.Q. Du, H. Ning, G.X. Li, W.L. Wei, Z.Q. Lan, and J. Guo, Catalytic effect of graphene on the hydrogen storage properties of Mg-Li alloy, Mater. Chem. Phys., 207(2018), p. 221.
J. Chen, Y.R. Li, L. Huang, C. Li, and G.Q. Shi, High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process, Carbon, 81(2015), p. 826.
M.J. Fernández-Merino, L. Guardia, J.I. Paredes, S. Villar-Rodil, P. Solis-Fernández, A. Martinez-Alonso, and J.M.D. Tascón, Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions, J. Phys. Chem. C, 114(2010), No. 14, p. 6426.
Y. Li, C.X. Wang, Z.T. Dong, J. Wang, S.Q. Yang, D.D. Ke, and S.M. Han, Effects of coating layers on electrochemical properties of Nd-Mg-Ni-based alloys, Int. J. Hydrogen Energy, 42(2017), No. 30, p. 19148.
H. Miao, M.X. Gao, Y.F. Liu, D. Zhu, and H.G. Pan, An improvement on cycling stability of Ti-V-Fe-based hydrogen storage alloys with Co substitution for Ni, J. Power Sources, 184(2008), No. 2, p. 627.
Y.F. Zhu, H.G. Pan, M.X. Gao, Y.F. Liu, and Q.D. Wang, Influence of annealing treatment on Laves phase compound containing a V-based BCC solid solution phase-Part II: Electrochemical properties, Int. J. Hydrogen Energy, 28(2003), No. 4, p. 395.
N. Kuriyama, T. Sakai, H. Miyamura, I. Uehara, H. Ishikawa, and T. Iwasaki, Electrochemical impedance and deterioration behavior of metal hydride electrodes, J. Alloys Compd., 202(1993), No. 1–2, p. 183.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NOs. 21303157 and 51771164), the Natural Science Foundation of Hebei Province (No. E2019203161), and Scientific Research Projects in Colleges and Universities in Hebei Province (No. QN2016002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Cheng, Ln., Miao, Wk. et al. Nd-Mg-Ni alloy electrodes modified by reduced graphene oxide with improved electrochemical kinetics. Int J Miner Metall Mater 27, 391–400 (2020). https://doi.org/10.1007/s12613-019-1880-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1880-z