Abstract
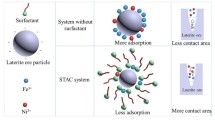
To solve the problem of low permeability and lower extraction rates of high-mud ores, a surfactant was added as a penetrant to the pregnant leaching solution during column leaching tests. On the basis of the theories of physical chemistry and seepage flow mechanics, the mechanism by which seepage is enhanced under the effects of the surfactant was analyzed. The results show that the action modes of the surfactant were divided into four aspects: changing the wettability of the ore, reducing the viscosity of the leaching solution, adsorbing onto the surface of ore, and enhancing the permeability effect. The findings of column leaching tests demonstrated that permeability was substantially improved by the surfactant. In the later period of leaching, the permeability coefficient was two times higher than that of the control group. Meanwhile, the ore extraction rate increased by approximately 10%. During the leaching process, the surface tension of the solution did not substantially change, and that of the solution with surfactant increased slightly. The kinetics analysis of ore column leaching illustrated that the leaching processes were controlled by both internal diffusion (principal factor) and chemical reaction.
Similar content being viewed by others
References
A.X. Wu, S.Y. Wang, S.H. Yin, and J.L. Yan, Chemical precipitation and control in process of heap leaching of high-alkali oxide copper ore, J. Cent. South Univ. Sci. Technol., 43(2012), No. 5, p. 1851.
J. Petersen, Heap leaching as a key technology for recovery of values from low-grade ores-A brief overview, Hydrometallurgy, 165(2016), p. 206.
J. Poisson, M. Chouteau, M. Aubertin, and D. Campos, Geophysical experiments to image the shallow internal structure and the moisture distribution of a mine waste rock pile, J. Appl. Geophys., 67(2009), No. 2, p. 179.
I.M.S.K. Ilankoon and S. J. Neethling, Liquid spread mechanisms in packed beds and heaps. The separation of length and time scales due to particle porosity, Miner. Eng., 86(2016), p. 130.
K. Quast, D.F. Xu, W. Skinner, A. Nosrati, T. Hilder, D.J. Robinson, and J. Addai-Mensah, Column leaching of nickel laterite agglomerates: Effect of feed size, Hydrometallurgy, 134(2013), p. 144.
Y.T. Wu, J. Meng, M.A. Chen, B.T. Fan, J.G. Zhang, and M. Ju, Study on permeation-promoter application in heap leaching process of uranium ores, Uranium Min. Metall., 26(2007), No. 2, p. 72.
B.T. Fan, J. Meng, Y.T. Wu, M.A. Chen, and Y.S. Meng, Improvement on uranium ore permeability with high molecular polymer surfactant, Hydrometall. China, 23(2004), No. 4, p. 211.
H.Z. Qi, K.X. Tan, S. Zeng, and J. Liu, Experimental study of in situ leaching uranium mining for low permeable sandstone uranium deposits using some surfactant, J. Nanhua Univ. Sci. Technol., 24(2010), No. 4, p. 19.
K.X. Tan, W.K. Dong, E.M. Hu, and Q.L. Wang, Preliminary study on improving permeability of ore-bearing layer using surfactant in in-situ leaching of uranium, Min. Res. Dev., 26(2006), No. 4, p. 10.
J.E. Waddell, M.J. Sierakowski, P.M. Savu, G.G. Moore, C.P. Jariwala, and M.A. Guerra, Leaching of Precious Metal Ore with Fluoroaliphatic Surfactant, U.S. Patent, Appl. 5612431, 1997.
A.A. Peng, H.C. Liu, Z.Y. Nie, and J.L. Xia, Effect of surfactant Tween-80 on sulfur oxidation and expression of sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans, Trans. Nonferrous Met. Soc. China, 22(2012), No. 12, p. 3147.
L.B. Luttinger, Erwinna, Recovery of Metal Values from Ores, U.S. Patent, Appl. 4929274, 1990
Q.C. Feng, W.J. Zhao, and S.M. Wen, Ammonia modification for enhancing adsorption of sulfide species onto malachite surfaces and implications for flotation, J. Alloys Compd., 744(2018), p. 301.
Q.C. Feng, W.J. Zhao, S.M. Wen, and Q.B. Cao, Copper sulfide species formed on malachite surfaces in relation to flotation, J. Ind. Eng. Chem., 48(2017), p. 125.
Q.C. Feng, W.J. Zhao, and S.M. Wen, Surface modification of malachite with ethanediamine and its effect on sulfidization flotation, Appl. Surf. Sci., 436(2018), p. 823.
C.M. Ai, A.X. Wu, Y.M. Wang, and C.L. Hou, Optimization and mechanism of surfactant accelerating leaching test, J. Cent. South Univ., 23(2016), No. 5, p. 1032.
P. Guo, S.J. Jiao, F. Chen, J. He, Y.Q. Li, and H. Zeng, Optimization and oil displacement efficiency of non-ionic low molecular surfactant, Oil Drill. Prod. Technol., 34(2012), No. 2, p. 81.
X.C. Fu, T. Chen, Z.M. Bao, R.J. Wang, and Y.N. Hu, Study on the wetting ability of class a foam extinguishing agent, Fire Sci. Technol., 29(2010), No. 2, p. 131.
J. Yang, Y.Z. Tan, Z.H. Wang, Y.D. Shang, and W.B. Zhao, Study on the coal dust surface characteristics and wetting mechanism, J. China Coal Soc., 32(2007), No. 7, p. 737.
A.X. Wu, C.M. Ai, Y.M. Wang, X.W. Li, Surfactant accelerating leaching of copper ores, J. Univ. Sci. Technol. Beijing, 35(2013), No. 6, p. 709.
H.M. Lizama, A kinetic description of percolation bioleaching, Miner. Eng., 17(2004), No. 1, p. 23.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51604138) and the Open Fund of the Key Laboratory of Ministry of Education of China for Efficient Mining and Safety of Metal Mines (No. ustbmslab201806).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ai, Cm., Sun, Pp., Wu, Ax. et al. Accelerating leaching of copper ore with surfactant and the analysis of reaction kinetics. Int J Miner Metall Mater 26, 274–281 (2019). https://doi.org/10.1007/s12613-019-1735-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1735-7