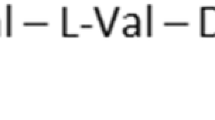Abstract
In this work, we performed the rational design of a cationic antimicrobial peptide, GIBIMPY4, using the software DEPRAMPs developed at the GIBIM research group. GIBIMPY4 has a length of 17 amino acids, it is amphipathic, its structure is α-helix and it has a net charge of (+5). Solid-phase peptide synthesis was performed using the Fmoc strategy in acid medium. The primary structure was confirmed by MALDI-TOF mass spectrometry. The antimicrobial activity of the peptide was evaluated by broth microdilution method by measuring optical density in 96-well microplates. The minimal inhibitory concentration of GIBIMPY4 to kill 50 % of the bacterial cells (MIC50) was 6.20 ± 0.02 µM for MRSA and 4.55 ± 0.02 µM for E. coli O157:H7, while also reporting a bacteriostatic effect for the later. GIBIMPY4 activity was sensitive to salt concentration in E. coli but insignificant effect in its activity against MRSA. The peptide seems to be a broad-spectrum antimicrobial agent based on the results against Gram-positive and Gram-negative bacteria and was specific for bacterial cells E. coli O157:H7 with index of specificity equal to 9.01 in vitro assays.


Similar content being viewed by others
References
World Health Organization (2014) Antimicrobial resistance: global report on surveillance. In: Surveillance of antimicrobial drug resistance in disease-specific programmes, 1st edn. WHO Library, Geneva, pp 1–256
Petty NK, Zakour NLB, Stanton-Cook M et al (2014) Global dissemination of a multidrug resistant Escherichia coli clone. Proc Natl Acad Sci USA 111:5694–5699. doi:10.1073/pnas.1322678111
Chambers HF (2001) The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis 7:178–182. doi:10.3201/eid0702.700178
Welte T, Pletz MW (2010) Antimicrobial treatment of nosocomial meticillin-resistant Staphylococcus aureus (MRSA) pneumonia: current and future options. Int J Antimicrob Agents 36:391–400. doi:10.1016/j.ijantimicag.2010.06.045
Haddadin AS, Fappiano SA, Lipsett PA (2002) Methicillian resistant Staphylococcus aureus (MRSA) in the intensive care unit. Postgrad Med J 78:385–392
Brogden KA (2011) Perspectives and peptides of the next generation. In: Drider D, Rebuffat S (eds) Prokaryotic antimicrobial peptides: genes to applications, 1st edn. Springer, New York, pp 423–440
Brandenburg LO, Merres J, Albrecht LJ et al (2012) Antimicrobial peptides: multifunctional drugs for different applications. Polymers 4:539–560. doi:10.3390/polym4010539
Baltzer SA, Brown MH (2011) Antimicrobial peptides—promising alternatives to conventional antibiotics. J Mol Microbiol Biotechnol 20:228–235. doi:10.1159/000331009
Hancock REW (2011) Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci 68:2161–2176. doi:10.1007/s00018-011-0710-x
Bhunia A, Saravanan R, Mohahram H et al (2011) NMR structures and interactions of Temporin-1Tl and Temporin-1Tb with lipopolysaccharide micelles. J Biol Chem 286:24394–24406. doi:10.1074/jbc.M110.189662
Rinaldi AC, Mangoni ML, Rufo A et al (2002) Temporin L: antimicrobial, haemolytic and cytotoxic activities, an effects on membrane permeabilization in lipid vesicles. J Biochem 368:91–100
Finberg RW, Moellering RC, Tally FP et al (2004) The importance of bactericidal drugs: future directions in infectious disease. Clin Infect Dis 39:1314–1320. doi:10.1086/425009
Sengupta D, Leontiadou H, Mark AE, Marrink SJ (2008) Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim Biophys Acta 1778:2308–2317. doi:10.1016/j.bbamem.2008.06.007
Shu JY, Huang YJ, Tan C et al (2010) Amphiphilic peptide-polymer conjugates based on the coiled-coil helix bundle. Biomacromolecules 11:1443–1452. doi:10.1021/bm100009e
Jahnsen RD, Frimodt-Møller N, Franzyk H (2012) Antimicrobial activity of peptidomimetics against multidrug-resistant Escherichia coli: a comparative study of different backbones. J Med Chem 55:7253–7261. doi:10.1021/jm300820a
Pitteloud J-P, Bionda N, Cudic P (2013) Synthesis of side chain N,N′-diaminoalkylated derivates of basic amino acids for application in solid-phase peptide synthesis. In: Cudic P (ed) Peptide modifications to increase metabolic stability and activity, 1st edn. Humana Press, New York, pp 211–236
Cheung WA, Hancock REW (2011) Optimization of antibacterial peptides by genetic algorithms and cheminformatics. Chem Biol Drug Des 77:48–56. doi:10.1111/j.1747-0285.2010.01044.x
Fjell CD, Hancock REW (2008) QSAR modeling and computer-aided design of antimicrobial peptides. J Pept Sci 14:110–114. doi:10.1002/psc
Dudek AZ, Arodz T, Galvez J (2006) Computational methods in developing quantitative structure-activity relationships (QSAR): a review. Comb Chem High Throughput Screen 9:213–228. doi:10.2174/138620706776055539
Thomas S, Karnik S, Barai RS et al (2009) CAMP: a useful resource for research on antimicrobial peptides. Nucl Acids Res 38:774–780. doi:10.1093/nar/gkp1021
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and Clustal X version 2.0. Bioinform Appl Note 23:2947–2948. doi:10.1093/bioinformatics/btm404
Thévenet P, Shen Y, Maupetit J et al (2012) PEP-FOLD: an updated de novo structure prediction server for both linear and disulfide bonded cyclic peptides. Nucl Acids Res 40:288–293. doi:10.1093/nar/gks419
Maupetit J, Derreumaux P, Tufféry P (2010) A fast method for large-scale de novo peptide and miniprotein structure prediction. J Comput Chem 31:726–738. doi:10.1002/jcc.21365
Keiderling TA (2002) Protein and peptide secondary structure and conformational determination with vibrational circular dichroism. Curr Opin Chem Biol 6:682–688
Cruz J, Ortiz C, Guzman F et al (2014) Design and activity of novel lactoferrampin analogues against O157:H7 enterohemorrhagic Escherichia coli. Biopolymers 101:319–328. doi:10.1002/bip.22360
Paredes D, Ortiz C, Torres R (2014) Synthesis, characterization, and evaluation of antibacterial effect of Ag nanoparticles against Escherichia coli O157:H7 and methicillin-resistant Staphylococcus aureus (MRSA). Int J Nanomed 9:1717–1729. doi:10.2147/IJN.S57156
Ma P, Wang Z, Pflugfelder S, Li D-Q (2010) Toll-like receptors mediate induction of peptidoglycan recognition proteins in human corneal epithelial cells. Exp Eye Res 90:130–136. doi:10.1016/j.exer.2009.09.021
Guan R, Roychowdhury A, Ember B et al (2004) Structural basis for peptidoglycan binding by peptidoglycan recognition proteins. PNAS 101:17168–17173
Gasteiger E, Hoogland C, Gattiker A et al (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook, 1st edn. Humana Press, Totowa, pp 571–607
Gopal R, Lee JK, Lee JH et al (2013) Effect of repetitive lysine-tryptophan motifs on the eukaryotic membrane. Amino Acids 44:645–660. doi:10.1007/s00726-012-1388-6
Mo RH, Zaro JL, Shen WC (2012) Comparison of cationic and amphipathic cell penetrating peptides for siRNA delivery and efficacy. Mol Pharm 9:299–309. doi:10.1021/mp200481g
Pandey BK, Srivastava S, Singh M, Ghosh JK (2011) Inducing toxicity by introducing a leucine-zipper-like motif in frog antimicrobial peptide, magainin 2. Biochem J 436:609–620. doi:10.1042/BJ20110056
Lohner K, Prenner EJ (1999) Differential scanning calorimetry and X-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochim Biophys Acta 1462:141–156. doi:10.1016/S0005-2736(99)00204-7
Slocik JM, Govorov AO, Naik RR (2011) Plasmonic circular dichroism of peptide-functionalized gold nanoparticles. Nano Lett 11:701–705. doi:10.1021/nl1038242
Gopal R, Park JS, Seo CH, Park Y (2012) Applications of circular dichroism for structural analysis of gelatin and antimicrobial peptides. Int J Mol Sci 13:3229–3244. doi:10.3390/ijms13033229
Wadhwani P, Reichert J, Ulrich AS (2012) Antimicrobial and cell-penetrating peptides induce lipid vesicle fusion by folding and aggregation. Eur Biophys J 41:177–187. doi:10.1007/s00249-011-0771-7
Bertsche U, Mayer C, Götz F, Gust AA (2014) Peptidoglycan perception—sensing bacteria by their common envelope structure. Int J Med Microbiol 305:217–223. doi:10.1016/j.ijmm.2014.12.019
Jacqueline C, Caillon J, Le Mabecque V et al (2003) In vitro activity of linezolid alone and in combination with gentamicin, vancomycin or rifampicin against methicillin-resistant Staphylococcus aureus by time-kill curve methods. J Antimicrob Chemother 51:857–864. doi:10.1093/jac/dkg160
Yu H, Tu C, Yip B et al (2011) Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob Agents Chemother 55:4918–4921. doi:10.1128/AAC.00202-11
Acknowledgments
The authors would like to thank The Administrative Department of Science, Technology and Innovation, COLCIENCIAS, for resources and funding provided for the development of this research. Also to the the Mass Spectrometry Laboratory of the Industrial University of Santander by the collection of mass spectra of the synthetic peptide.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prada, Y.A., Guzmán, F., Rondón, P. et al. A New Synthetic Peptide with In vitro Antibacterial Potential Against Escherichia coli O157:H7 and Methicillin-Resistant Staphylococcus aureus (MRSA). Probiotics & Antimicro. Prot. 8, 134–140 (2016). https://doi.org/10.1007/s12602-016-9219-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-016-9219-9