Abstract
We examined the roles of aortic and carotid sinus baroreceptors in control of heart rate (HR) and renal sympathetic nerve activity (RSNA) in 17 decerebrate rats. The baroreflex curves between the changes in mean arterial blood pressure (MAP) and HR or RSNA in response to intravenous injection of phenylephrine (10–20 μg/kg) or nitroprusside (10 μg/kg) were identified before and following sequential denervation of all four baroafferent nerves. The slope of the MAP–HR curve in the pressor range was decreased (P < 0.05) to 31 ± 7 % of the control following denervation of bilateral aortic nerves, whereas it remained substantial (72 ± 10 %) following denervation of bilateral carotid sinus nerves. The slope for HR became negligible following complete denervation of all four baroafferent nerves. In contrast, the slope of the MAP–RSNA curve decreased as the sequential baroafferent denervation progressed, irrespective of the denervation order, and it remained well as long as any single baroafferent nerve was intact. The similar influences of sequential baroafferent denervation on the responses of HR and RSNA were observed in the depressor range. Thus, it is likely that aortic and carotid sinus baroreceptors play differential roles in control of HR but they contribute similarly to control of RSNA.
Similar content being viewed by others
Introduction
Aortic and carotid sinus baroreceptors are mechanically sensitive to transmural pressures of the aortic arch and carotid sinus and constitute aortic and carotid sinus baroreflex, respectively. Both types of arterial baroreceptors convey information about beat-by-beat blood pressure to the central nervous system and regulate vascular resistance and cardiac output to help mean arterial blood pressure (MAP) remain constant.
Aortic and carotid sinus baroreflexes may play different roles in control of the vasomotor and cardiac functions. Previous studies found that baroreflex control of renal sympathetic nerve activity (RSNA) and hindlimb vascular resistance is achieved by mutually inhibitory summation of inputs from both aortic and carotid sinus baroafferents in anesthetized animals [1–4]. However, it is important to evaluate the baroreflex function in the unanesthetized condition because anesthesia alters the gain and other characteristics of arterial baroreflexes [5–7]. On the other hand, it has been reported that the baroreflex control of cardiac function chiefly derives from aortic baroafferents with relatively little contribution of carotid sinus baroafferents in humans and unanesthetized animals [8–10]. Pickering et al. [11] examined the different roles of aortic and carotid sinus baroreflexes in control for heart rate (HR) and sympathetic nerve activity using sequential baroafferent denervation in artificially perfused in situ decerebrate preparations of the immature rat (postnatal age 28–35 days). They found that baroreflex control of HR is predominantly accomplished by aortic baroafferents, whereas both aortic and carotid sinus baroafferents equally contribute to baroreflexly evoked sympathoinhibition. However, the special in situ preparation, in which they removed the stomach, intestines, and spleen and perfused artificially with static pressure, is clearly different from the in vivo condition. The evidence obtained from the special in situ preparation may not always be true in the unanesthetized in vivo preparation. Furthermore, it is known that baroreflex sensitivities for cardiac and vasomotor limbs are decreased with maturation and aging [12]. Electrical stimulation of the left aortic baroafferent caused weaker baroreflex bradycardia and sympathetic inhibition in 9-month-old rats than in 2-month-old rats [13], suggesting that control of cardiac and vasomotor functions by aortic baroreflex may differ between immature young and adult animals. Accordingly, we have reexamined the roles of aortic and carotid sinus baroafferents in control of HR and RSNA by sequential denervation of the four baroafferent nerves using unanesthetized decerebrate in vivo preparations of the adult rat. A part of this study has been preliminarily reported [14].
Methods
The present study was performed using 17 male Wistar adult rats (456 ± 22 g, 11–26 weeks) in accordance with the “Guiding principles for the care and use of animals in the fields of physiology sciences” approved by the Physiological Society of Japan and the Guidelines for Animal Experiments in Hiroshima University. The experimental protocols and procedures were approved by the Committee of Research Facilities for Laboratory Animal Science, Natural Science Center for Basic Research and Development, Hiroshima University.
Preparations
The rats were anesthetized by inhaling a gas mixture of halothane (4 %), N2O (0.5 l/min), and O2 (0.5 l/min) in a small box and then an endotracheal tube was inserted into the airway. Surgical anesthesia was maintained with a halothane (0.5–1.0 %)–N2O–O2 gas mixture through the endotracheal tube to implant catheters, perform decerebration surgery, and isolate the renal nerve. The lungs were ventilated by an artificial ventilator (model SN-480-7, Shinano, Tokyo, Japan). An electrocardiogram (ECG), HR, rectal temperature, and thoracic respiratory movement were continuously monitored throughout the experiments. HR was derived from the R wave of ECG with a tachometer (model 1321, GE Marquette Medical Systems, Tokyo, Japan). Rectal temperature was maintained at 37–38 °C with a heating pad and a lamp. To maintain an appropriate level of anesthesia during surgery, the concentration of halothane was increased to 1.5–2.0 % if HR and/or respiration increased and/or if limb withdrawal occurred in response to a noxious pinch of the paw. One polyvinyl catheter was inserted into the left brachial vein for administering drugs, and another one was inserted into the left brachial artery and further progressed toward the aorta for measuring arterial blood pressure (AP). The arterial catheter was connected to a pressure transducer (DPT-6100, Kawasumi Laboratories, Tokyo, Japan). The head of the rat was then mounted on a stereotaxic frame. Decerebration was performed by electrocoagulation at the precollicular–premammillary level. To do this, a stainless steel electrode with insulation removed 5 mm from the tip was inserted into the hypothalamus to the mamillary bodies (coordinates from the bregma: posterior 4.0 mm, horizontal 9.5 mm, lateral 0.5–5.0 mm; from a stereotaxic atlas [15]). A negative DC current (1 mA) was passed through the electrode for 30 s. The electrode was withdrawn 4 mm and the current was passed again. This procedure was bilaterally repeated for a total of 20 tracks at 0.5 mm intervals. After the decerebration was completed, the rats were removed from the stereotaxic frame and placed in a lateral posture. The left kidney was exposed via a retroperitoneal approach. A renal nerve bundle was separated from the renal plexus and surrounding connective tissue near the renal artery and vein using an operating microscope. Multifiber RSNA was measured using a bipolar electrode of Teflon-coated Ag wires and amplified by a differential preamplifier (MEG-2100, Nihon Kohden, Tokyo, Japan) with a band-pass filter of 50–3000 Hz. The nerve-electrode complex was covered with silicone gel for insulation. The amplified and filtered output of RSNA was sampled at a frequency of 10 kHz and the spike peaks in the RSNA were converted to standard pulse trains using a digital technique, which detected the peaks of the original nerve signals [16]. The pulse trains were integrated by a resistance–capacitance integrator with a time constant of 20 ms.
Protocols
After all surgical and preparatory procedures were completed, inhalation anesthesia was stopped and a neuromuscular blocker (d-tubocurarine, 0.5 mg) was administered intravenously. The experiments were started 2–3 h after cessation of the anesthesia. To identify the stimulus–response relationship between MAP and HR or RSNA, phenylephrine (10–20 μg/kg) and nitroprusside (10 μg/kg) were administered intravenously in the intact baroafferent condition. Then the aortic nerve (AoN) was bilaterally identified between the cervical vagal and sympathetic nerves, and the carotid sinus nerve (CSN) was bilaterally identified in the carotid sinus region. The roles of aortic and carotid sinus baroreflexes in control of HR and RSNA during the pharmacological interventions were examined by conducting sequential denervation of the four baroafferent nerves. The sequential denervation of AoN was followed by that of CSN in ten rats (AoN–CSN group); RSNA was measured in seven of the ten rats. The order of the sequential denervation was reversed in another seven rats (CSN–AoN group); RSNA was measured in five of the seven rats. The phenylephrine challenge was performed in the first series of the experiments using seven rats (four rats in AoN–CSN group and three rats in CSN–AoN group). In the second series of the experiments, both phenylephrine and nitroprusside challenges were performed in ten rats (six rats in AoN–CSN group and four rats in CSN–AoN group). The rats were killed with an overdose of pentobarbital sodium at the end of the experiments and the transected area of the brain was examined histologically. We confirmed that the cerebral cortex, the thalamus, and a rostral part of the hypothalamus (the anterior hypothalamic area, the supraoptic nucleus, and the rostral part of the lateral hypothalamus area) were disconnected from the brain stem.
Data treatment and statistical analysis
ECG, HR, AP and original and integrated RSNA were continuously recorded on an eight-channel pen-writing recorder (Recti-8 K, GE Marquette Medical Systems, Tokyo, Japan). The data were stored in a computer with an analog-to-digital converter (MP150, BIOPACK Systems, Santa Barbara, CA, USA) at a sampling frequency of 2000 Hz for off-line analysis. HR, AP, and integrated RSNA were sequentially averaged over neighboring 2000 points. The averaged AP value was defined as MAP. The HR values in response to the pharmacological interventions were collected, taking into account a delay time of 4–12 beats (average 6 ± 0.2 beats) from the MAP change to the baroreflex response in HR. The baseline levels of HR, MAP, and integrated RSNA were defined as the mean values for more than 30 beats preceding the pharmacological interventions. The changes in HR, MAP, and RSNA from the baseline levels during the pharmacological interventions were used to construct the stimulus–response curves between HR or RSNA and MAP every 5 mmHg. The magnitude of RSNA response in a given trial was relatively expressed as a percentage against the baseline value, which was taken as 100 % in each condition. The slope of the baroreflex curves was calculated with a regression method, as an estimate of the baroreflex sensitivity, and further averaged among the animals. To cancel inter-animal variability of the baroreflex slope, the normalized relative slopes of the baroreflex curves were calculated as a ratio to the slope in the intact baroafferent condition.
The baseline values after sequential baroafferent denervation were compared with those in the intact condition using a one-way ANOVA with repeated measures followed by a post hoc test of Dunnett. The baroreflex sensitivity was analyzed using a two-way ANOVA (denervation order × the number of denervated baroafferent nerves). If an interaction between the two factors was significant, the Dunnett post hoc test was performed. If the significant interaction between the two factors was absent and the main effect of the number of the denervated baroafferent nerves was significant, a one-way ANOVA with repeated measures was performed with the Dunnett post hoc test. All statistical procedures were conducted using SigmaPlot® version 12.5 (Systat Software, San Jose, CA). A level of statistical significance was defined at P < 0.05 in all cases. All parameters are expressed as mean ± SE.
Results
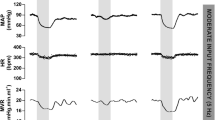
The baseline values of HR and RSNA increased with progress of denervation of the baroafferents in both AoN–CSN and CSN–AoN groups (Table 1; Fig. 1). The baseline MAP was not significantly (P > 0.05) affected by the sequential denervation.
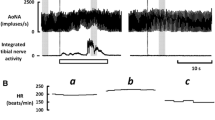
The influence of sequential baroafferent denervation on the baroreflex bradycardia and sympathoinhibition evoked by the phenylephrine-induced pressor response. a Sequential denervation of the aortic nerves followed by denervation of the carotid sinus nerves (AoN–CSN) in one rat. b Sequential denervation of the carotid sinus nerves followed by denervation of the aortic nerves (CSN–AoN) in another rat. ‘Intact’, 1st, 2nd, 3rd, and ‘all’ mean that the number of the denervated baroafferent nerves was zero, one, two, three, and four, respectively. ‘Averaged RSNA’ shows moving averages of the integrated RSNA. The baroreflex bradycardia was obviously blunted by single denervation of the aortic nerve irrespective of the AoN–CSN or CSN–AoN group, whereas the baroreflex inhibition of RSNA was well preserved as long as a single aortic or carotid sinus nerve remained intact. Arrhythmia was sometimes observed in the intact and 3rd condition of the CSN–AoN. HR heart rate, AP arterial blood pressure, RSNA renal sympathetic nerve activity
An increase in AP by phenylephrine caused the baroreflex decreases in HR and RSNA in the intact condition as shown in Fig. 1. Unilateral denervation of the AoN attenuated substantially the baroreflex bradycardia with little effect on the baroreflex inhibition of RSNA (Fig. 1a). Even denervation of both AoN and unilateral CSN did not attenuate the RSNA response, which was abolished by the denervation of all baroafferent nerves. On the other hand, the reverse order of barodenervation showed that denervation of bilateral CSN seemed not to affect the decrease responses of HR and RSNA (Fig. 1b). However, subsequent 3rd denervation of unilateral AoN abolished the baroreflex bradycardia without altering the baroreflex inhibition of RSNA. The RSNA inhibition was abolished by the denervation of all four baroafferent nerves.
Effect of sequential baroafferent denervation on the MAP–HR curve
Figure 2a represents the stimulus–response curves of the baroreflex changes in HR in response to the pharmacological pressor and depressor interventions. In the AoN–CSN group, the slope of the MAP–HR curve in the pressor range reduced significantly (P < 0.05) following sequential AoN denervation as compared to that in the intact condition (Figs. 2, 3a). The relative slope of the MAP–HR curve decreased by 35 ± 5 % of the control slope following unilateral AoN denervation and further by 35 ± 6 % following bilateral AoN denervation (Fig. 4a). The reduced slope of the MAP–HR curve was abolished by subsequent CSN denervation.
The influences of sequential baroafferent denervation on the stimulus–response curves between the average changes in mean arterial blood pressure (MAP) and HR (a) or RSNA (b) in response to phenylephrine and nitroprusside. The magnitude of the RSNA response in a given bout was expressed as a percentage against the baseline value, which was taken as 100 % in each condition. AoN–CSN; sequential denervation of the aortic nerves followed by denervation of the carotid sinus nerves. CSN–AoN; sequential denervation of the carotid sinus nerves followed by denervation of the aortic nerves
The influences of sequential baroafferent denervation on the absolute slopes of the ΔMAP–ΔHR curves (a) and the ΔMAP–ΔRSNA curves (b) during the phenylephrine- or nitroprusside-induced intervention. The slopes of the ΔMAP–ΔHR and ΔMAP–ΔRSNA curves in both AoN–CSN and CSN–AoN groups were analyzed by a two-way ANOVA (denervation order × the number of denervated baroafferent nerves). Since a significant interaction between the two factors was absent and the main effect of the number of the denervated nerves was significant, a one-way ANOVA with repeated measures was performed with the Dunnett post hoc test. *Significant difference (P < 0.05) from the value in the intact condition
The influences of sequential baroafferent denervation on the normalized relative slopes of the ΔMAP–ΔHR curves (a) and the ΔMAP–ΔRSNA curves (b) during the phenylephrine- or nitroprusside-induced intervention. The relative slopes of the baroreflex curves were calculated as a ratio to the control slope in the intact condition. The relative slopes of the ΔMAP–ΔHR and ΔMAP–ΔRSNA curves in both AoN–CSN and CSN–AoN groups were analyzed by a two-way ANOVA (denervation order × the number of denervated baroafferent nerves). When an interaction between the two factors was significant, the Dunnett post hoc test was performed. When a significant interaction between the two factors was absent and the main effect of the number of the denervated nerves was significant, a one-way ANOVA with repeated measures was performed with the Dunnett post hoc test. *Significant difference (P < 0.05) from the value in the intact condition. †Significant difference (P < 0.05) between the AoN–CSN and the CSN–AoN groups
In contrast, in the CSN–AoN group, the slope of the MAP–HR curve in the pressor range was not greatly affected by sequential CSN denervation (Figs. 2a, 3). The relative slope of the MAP–HR curve was not significantly altered following denervation of unilateral CSN but decreased (P < 0.05) by 28 ± 10 % from the control slope following bilateral CSN denervation (Fig. 4). Thus, the relative slope of the MAP–HR curves substantially remained (P < 0.05) following the CSN denervation (72 ± 10 % of the control slope) as compared to the AoN denervation in the AoN–CSN group (31 ± 7 % of the control slope), indicating a greater role of aortic baroreflex on HR than carotid sinus baroreflex. Such significant difference in the relative slopes of the MAP–HR curves was seen even after the 3rd denervation (single AoN vs CSN). The denervation of all four baroafferents almost abolished the relative baroreflex slope (Fig. 4).
The slopes of the MAP–HR curves in the depressor range were much smaller than those in the pressor range (Figs. 2a, 3). As observed in the pressor range, there was a tendency that the baroreflex slope in the depressor range was differentially affected by sequential barodenervation between the AoN–CSN and CSN–AoN groups, although the main effect of denervation order on the baroreflex slope was not significant (Fig. 4).
Effect of sequential baroafferent denervation on the MAP–RSNA curve
The baroreflex inhibition and activation of RSNA in response to the pressor and depressor interventions composed an inverse sigmoidal stimulus–response curve between MAP and RSNA in the intact condition (Fig. 2b). Although the denervation order had a significant influence on the slope of the MAP–RSNA curve (Fig. 3b), the comparison of the relative slopes of the MAP–RSNA curves between the two groups showed no significant effect of the denervation order on the baroreflex slope (Fig. 4b). The relative slope of the MAP–RSNA curve in the pressor range decreased (P > 0.05) to 59 ± 12 % of the control slope following denervation of bilateral AoN in the AoN–CSN group, whereas the relative slope tended to decrease (87 ± 12 % of the control slope) following CSN denervation in the CSN–AoN group. Accordingly, the size of the intact baroreflex sensitivity for RSNA became smaller than the simple algebraic sum of the individual sensitivity of aortic and carotid sinus baroreflexes. In either group, 54–55 % of the relative slopes of the MAP–RSNA curves was preserved as long as a single AoN or CSN remained intact. The denervation of all four baroafferent nerves reduced the relative slope of MAP–RSNA curve to 16–20 %.
Like the cardiac baroreflex curve, the slopes of the MAP–RSNA curves in the depressor range were much smaller than those in the pressor range (Figs. 2, 3). The baroreflex slope in the depressor range tended to decrease according to the number of denervated baroafferent nerves, irrespective of the denervation order, and was almost abolished (P < 0.05) by denervation of all baroafferent nerves (Figs. 3, 4).
Discussion
The present study has tested the hypothesis that aortic and carotid baroafferents have different functional roles in control of HR and RSNA. To do this, we have examined the influences of sequential baroafferent denervation on the responses in HR and RSNA during the pharmacologically induced pressor and depressor interventions in unanesthetized, decerebrate adult rats. The major new findings of this study are that (1) the baroreflex sensitivity for HR was substantially decreased by AoN denervation rather than CSN denervation; (2) approximately 60 % of the baroreflex sensitivity for RSNA was preserved as long as any single baroafferent remained intact, irrespective of the denervation order. Taken together, it is likely that aortic and carotid sinus baroafferents play differential roles in control of HR but contribute equally to control of RSNA.
Some substantial limitations are involved in this study. First, since the order of sequential denervation of left and right baroafferent nerves was not completely randomized, the laterality difference in the baroreflex control is still unclear. Second, careful consideration is needed to evaluate the baroreflex sensitivity for HR in the depressor range, because the operating point of the baroreceptor-cardiac reflex was near the upper plateau of the stimulus–response curve as observed previously in conscious rats [17]. The pharmacological depressor intervention caused a slight tachycardiac response, resulting in difficulty in evaluating the baroreflex slope (Fig. 2). In addition, great variability of the baroreflex tachycardiac response was found, probably due to the small sample size. However, since the baroreflex sensitivity for HR in the depressor range approximately doubled in the intact AoN alone as compared to the intact CSN alone, we believe that aortic baroreflex has greater contribution to the baroreflex tachycardia than carotid sinus baroreflex. Third, the baroreflex response of RSNA was expressed as a percentage against the 100 % baseline level, because the baseline RSNA varied among animals. In addition, we could not always compare the absolute responses of RSNA among the conditions in a given animal, because it was difficult to keep the recording condition of RSNA in good shape throughout the experiment. Such good recording of RSNA obtained in six rats revealed that the sequential barodenervation resulted in the increase in baseline RSNA and a rightward shift of the point reaching the lower plateau of the stimulus–response curve to a higher pressure (Fig. 5). Thus, it is likely that the slope of the MAP–RSNA curve (i.e., baroreflex sensitivity for RSNA) was preserved well as long as any single baroafferent nerve remained intact, irrespective of expressing the RSNA response as an absolute value or percentage. It is still unknown why the reduced number of baroreceptor inputs preserved the baroreflex sensitivity for RSNA. Fourth, the range of ages in rats used in this study was wide (11–26 weeks), although it was found that there were no significant correlations between the baroreflex sensitivity for HR or RSNA and their ages.
The influences of sequential baroafferent denervation on the stimulus–response curves between the changes in MAP and the absolute RSNA values in response to phenylephrine and nitroprusside. a Sequential denervation of the aortic nerves followed by denervation of the carotid sinus nerves (AoN–CSN) in one rat. b Sequential denervation of the carotid sinus nerves followed by denervation of the aortic nerves (CSN–AoN) in another rat. The RSNA value at the point (∆MAP = −10 mmHg) in the intact condition of the CSN–AoN rat was removed due to involvement of an artifact
The dominant role of aortic baroreflex in the control of HR is supported by previous studies using decerebrate, artificially perfused in situ preparations of the immature young rat [11], conscious rabbit [8], and man [9]. The functional difference in control of HR between aortic and carotid sinus baroreflex may be due to a difference in input signal from arterial baroreceptors in each region. Matsukawa et al. [18], however, reported that the mean activity of the AoN and CSN increased similarly by 78–81 % and by 81 % of the baseline control during a rise in MAP of 34–40 mmHg in decerebrate cats, suggesting that afferent signals from aortic and carotid sinus baroreceptors are transmitted to the central nervous system to the same extent. Thus, it is likely that the central baroreflex pathway within the brain stem differs between aortic and carotid sinus baroreflexes. Although primary aortic and carotid sinus baroafferents project to the same region of the nucleus tractus solitarius (NTS), some of the two baroafferents distribute differentially to subgroups of the NTS [19–23]. Furthermore, since the baroreflex bradycardia in response to stimulation of the AoN was reduced by propranolol to 73 % of the control and the remaining bradycardia was abolished by subsequent atropine in decerebrate cats [24], aortic baroreflexly induced bradycardia is predominantly evoked by activation of the cardiac parasympathetic nerve. However, whether this notion is true in the rat remains to be studied, because there may be species differences in baroreflex control of HR. Taking these findings into consideration, it is possible that NTS neurons targeted by the AoN may constitute a discrete pathway of the cardiac baroreflex, dominantly controlling HR through cardiac parasympathetic outflow.
We found that the size of the intact baroreflex sensitivity for HR (approximately −1.4 beats/min/mmHg) was almost the same as the algebraic sum of the individual sensitivities of aortic and carotid sinus baroreflexes, suggesting that aortic and carotid sinus baroreflexes regulate HR independently in a simple summative manner in decerebrate adult rats. On the other hand, Pickering et al. [11] reported a greater baroreflex sensitivity for HR (approximately −2.6 beats/min/mmHg), which was achieved by a facilitatory interaction between aortic baroafferents in decerebrate preparations of the immature young rat. These results lead to a hypothesis that the changes in the central nervous system in association with maturation may cause the decrease in aortic baroreflex sensitivity for HR, which is counteracted by an additional recruitment of carotid sinus baroreflex. This hypothesis is partly supported by the previous finding that a weaker baroreflex bradycardia and sympathetic inhibition is caused by electrical stimulation of the left aortic baroafferent in 9-month-old rats compared with 2-month-old rats [13].
In regard to the baroreflex regulation of RSNA, the present study using the decerebrate adult rats demonstrated that both aortic and carotid sinus baroreflexes cooperate to regulate RSNA in a mutually inhibitory manner as reported previously [1, 2, 4, 5]. A similar interaction was also observed in the baroreflex regulation of superior mesenteric nerve activity in artificially perfused decerebrate rat preparations [11], although the size of the baroreflex sensitivity for sympathetic mesenteric nerve activity was smaller than the baroreflex sensitivity for RSNA. In contrast to the cardiac pathways, vasomotor pathways of aortic and carotid sinus baroreflexes may not be modulated with maturation. Since the pressure-encoding properties of baroreceptors are probably similar between aortic and carotid sinus baroreceptors, as mentioned above, the mutually inhibitory control of RSNA by the two baroreflexes may originate in the central pathway of the baroreflex; for example, the NTS neurons receiving convergent inputs from the AoN and CSN [19–23]. It was noted that the baroreflex inhibition of RSNA evoked by the pressor interventions was still observed after denervation of all baroafferents. The remaining inhibition of RSNA may be caused by the signals from cardiopulmonary and other baroreceptors through vagal afferents [3].
It is expected that the differential control of HR and RSNA by aortic and carotid sinus baroreflexes is beneficial for cardiovascular regulation during exercise. Our laboratory reported that central command blunts the sensitivity of the cardiac component of the aortic baroreflex at the onset of spontaneous motor activity, preserving the cardiac sensitivity of the carotid sinus baroreflex [10, 25]. Since the aortic baroreflex has a dominant role in HR control, it is likely that selective suppression of aortic baroreflex gain by central command contributes to an instantaneous increase in HR at the onset of exercise, despite a concomitant rise in MAP. As a result, if a greater elevation in AP is evoked during exercise, the carotid sinus baroreflex preserving the baroreflex sensitivity may cause baroreflex bradycardia to some extent. Furthermore, since the sensitivity of the vasomotor component of both aortic and carotid sinus baroreflexes is also preserved during exercise [24, 26], the increase in AP is counteracted by a reduction in total peripheral resistance. Thus, preservation of both vasomotor and cardiac components of the carotid sinus baroreflex during exercise may have an important physiological significance in preventing an excess elevation in AP during exercise [10].
This study has shown the differential contribution of aortic and carotid sinus baroafferents to the control of HR and the sympathetic outflow in decerebrate adult rats. It is concluded that aortic baroreflex plays a more dominant role in the reflex control of HR, whereas both aortic and carotid sinus baroreflexes contribute redundantly to baroreflex regulation of sympathetic outflows to blood vessels.
References
Sagawa K, Watanabe K (1965) Summation of bilateral carotid sinus signals in the barostatic reflex. Am J Physiol 209:1278–1286
Ninomiya I, Irisawa I (1969) Summation of baroreceptor reflex effects on sympathetic nerve activities. Am J Physiol 216:1330–1378
Guo GB, Thames MD, Abboud FM (1982) Differential baroreflex control of heart rate and vascular resistance in rabbits: relative role of carotid, aortic, and cardiopulmonary baroreceptors. Circ Res 50:554–565
Thames MD, Ballon BJ (1984) Occlusive summation of carotid and aortic baroreflexes in control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 246:H851–H857
Sagawa K (1983) Baroreflex control of systemic arterial pressure and vascular bed. In: Shepherd JT, Abboud FM, Geiger SR (eds) Handbook of physiology: the cardiovascular system, vol 3. APS, Bethesda, pp 453–496
Matsukawa K, Ninomiya I (1989) Anesthetic effects on tonic and reflex renal sympathetic nerve activity in awake cats. Am J Physiol Regul Integr Comp Physiol 256:R371–R378
Matsukawa K, Ninomiya I, Nishiura N (1993) Effects of anesthesia on cardiac and renal sympathetic nerve activities and plasma catecholamines. Am J Physiol Regul Integr Comp Physiol 265:R792–R797
Alexander N, De Cuir M (1963) Role of aortic and vagus nerves in arterial baroreflex bradycardia in rabbits. Am J Physiol 205:775–780
Ferguson DW, Abboud FM, Mark AL (1985) Relative contribution of aortic and carotid baroreflexes to heart rate control in man during steady state and dynamic increases in arterial pressure. J Clin Invest 76:2265–2274
Matsukawa K, Ishii K, Idesako M, Ishida T, Endo K, Liang N (2013) Central command differentially affects aortic and carotid sinus baroreflexes at the onset of spontaneous motor activity. Auton Neurosci 179:75–83
Pickering AE, Simms AE, Paton JF (2008) Dominant role of aortic baroreceptors in the cardiac baroreflex of the rat in situ. Auton Neurosci 142:32–39
Segar JL (1997) Ontogeny of the arterial and cardiopulmonary baroreflex during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 273:R457–R471
Tanabe S, Buñag RD (1989) Age-related central and baroreceptor impairment in female Sprague-Dawley rats. Am J Physiol Heart Circ Physiol 256:H1399–H1406
Idesako M, Matsukawa K, Ishii K, Endo K, Liang N (2014) Aortic baroreceptors play a greater role in baroreflex regulation of heart rate than carotid sinus baroreceptors in unanesthetized, decerebrate rats. J Physiol Sci 64(suppl 1):S125
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Ninomiya I, Matsukawa K, Honda T, Nishiura N, Shirai M (1986) Cardiac sympathetic nerve activity and heart rate during coronary occlusion in awake cats. Am J Physiol Heart Circ Physiol 251:H528–H537
Miki K, Yoshimoto M, Tanimizu M (2003) Acute shifts of baroreflex control of renal sympathetic nerve activity induced by treadmill exercise in rats. J Physiol 548:313–322
Matsukawa K, Ishii K, Kadowaki A, Ishida T, Idesako M, Liang N (2014) Discharges of aortic and carotid sinus baroreceptors during spontaneous motor activity and pharmacologically evoked pressor interventions. J Physiol Sci 64:291–303
Ciriello J, Calaresu FR (1981) Projections from buffer nerves to the nucleus of the solitary tract: an anatomical and electrophysiological study in the cat. J Auton Nerv Syst 3:299–310
Donoghue S, Felder RB, Gilbey MP, Jordan D, Spyer KM (1985) Post-synaptic activity evoked in the nucleus tractus solitarius by carotid sinus and aortic nerve afferents in the cat. J Physiol 360:261–273
Paton JF, Silva-Carvalho L, Goldsmith GE, Spyer KM (2001) Inhibition of barosensitive neurons evoked by lobule IXb of the posterior cerebellar cortex in the decerebrate rabbit. J Physiol 427:553–565
Nosaka S, Murase S, Murata K, Inui K (1995) ‘Aortic baroreceptor’ neurons in the nucleus tractus solitarius in rats: convergence of cardiovascular inputs as revealed by heartbeat-locked activity. J Auton Nerv Syst 55:69–80
Chan RK, Jarvina EV, Sawchenko PE (2000) Effects of selective sinoaortic denervations on phenylephrine-induced activational responses in the nucleus of the solitary tract. Neuroscience 10:165–178
Murata J, Matsukawa K, Komine H, Tsuchimochi H, Nakamoto T (2004) Central inhibition of the aortic baroreceptors-heart rate reflex at the onset of spontaneous muscle contraction. J Appl Physiol 97:1371–1378
Matsukawa K, Ishii K, Kadowaki A, Liang N, Ishida T (2012) Differential effect of central command on aortic and carotid sinus baroreceptor-heart rate reflexes at the onset of spontaneous, fictive motor activity. Am J Physiol Heart Circ Phsyiol 303:H464–H474
Komine H, Matsukawa K, Tsuchimochi H, Murata J (2003) Central command blunts the baroreflex bradycardia to aortic nerve stimulation at the onset of voluntary static exercise in cats. Am J Physiol Heart Circ Physiol 285:H516–H526
Acknowledgments
We really appreciate the valuable expert advice of Dr. Minoru Shinohara (Georgia Institute of Technology) in reviewing the manuscript. This study was supported by Grants-in-Aid for Scientific Research (B) and for Exploratory Research from the Japan Society for the Promotion of Science (JSPS) and for JSPS Fellows (K.I.).
Author contribution
K.M. designed the experiments; K.I., I.M., and K.M. performed the experiments; K.I. and I.M. analyzed the data; K.I. and K.M. drafted the article; K.M. revised the article critically for intellectual content. All authors approved the final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ishii, K., Mitsuhiro, I. & Matsukawa, K. Differential contribution of aortic and carotid sinus baroreflexes to control of heart rate and renal sympathetic nerve activity. J Physiol Sci 65, 471–480 (2015). https://doi.org/10.1007/s12576-015-0387-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-015-0387-2