Abstract
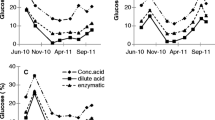
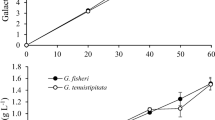
This study compared the ethanol productivity of different yeast strains using three different seaweed species as fermentation feedstocks. Each seaweed was saccharified by treatment with 3 % (v/v) sulfuric acid and cellulase. Ulva spp., Gracilaria spp. and Costaria costata yielded 0.22, 0.16, and 0.10 g of reducing sugars per 1 g of dried seaweed powder, respectively. Among the yeast strains tested, the marine-derived C-19 had maximum ethanol productivity, with production of 0.15, 0.08, and 0.05 g of ethanol from saccharified solutions containing 1 g of Ulva spp., Gracilaria spp., and C. costata powder, respectively. By the optimization of pretreatment, saccharification and fermentation conditions, C-19 yeast became capable of producing 0.09 g of ethanol from the alginate-extracted residue of C. costata. Finally, to evaluate the robustness of our measurements, we performed scale-up saccharification and fermentation using a jar fermentor. The productivity of both saccharification and fermentation were similar across both scales. Thus, we confirmed that the ethanol fermentation conditions from seaweeds powders in this study are appropriate even at larger scales.








Similar content being viewed by others
References
Quintero JA, Montoya MI, Sanchez OJ, Sanchez OJ, Giraldo OH, Giraldo OH (2008) Fuel ethanol production from sugarcane and corn: comparative analysis for a Colombian case. Energy (Oxf) 33:385–399
Wang R, Dominguez-Espinosa RM, Leonard K, Konutinas A, Webb C (2002) The application of a generic feedstock from wheat for microbial fermentation. Biotechnol Prog 18:1033–1038
Ogbonna JC, Mashima H, Tanaka H (2001) Scale up fuel ethanol production from sugar beet juice using loofa sponge immobilized bioreactor. Bioresour Technol 76:1–8
Velmurugan R, Muthukumar K (2011) Utilization of sugarcane bagasse for bioethanol production: sono-assisted acid hydrolysis approach. Bioresour Technol 102:7119–7123
Okamoto K, Nitta Y, Maekawa N, Yanase H (2011) Direct ethanol production from starch, wheat bran and rice straw by the white rot fungus Trametes hirsuta. Enzyme Microb Technol 48:273–277
Shinozaki Y, Kitamoto HK (2011) Ethanol production from ensiled rice straw and whole-crop silage by the simultaneous enzymatic saccharification and fermentation process. J Biosci Bioeng 111:320–325
Wargacki AJ, Leonard E, Win MN, Regitsky DD, Santos CN, Kim PB, Cooper SR, Raisner RM, Herman A, Sivitz AB, Lakshmanaswamy A, Kashiyama Y, Baker D, Yoshikuni Y (2012) An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335:308–313
Ross AB, Jones JM, Kubacki ML, Bridgeman T (2008) Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour Technol 99:6494–6504
Demirbas A (2010) Use of algae as biofuel sources. Energy Convers Manag 51:2738–2749
Obara N, Ishida M, Hamada-sato N, Urano N (2012) Efficient bioethanol production from paper shredder scrap by a marine derived Saccharomyces cerevisiae C-19. Stud Sci Technol 1:49–54
Takagi T, Uchida M, Matsushima R, Ishida M, Urano N (2012) Efficient bioethanol production from water hyacinth Eichhornia crassipes by both preparation of the saccharified solution and selection of fermenting yeasts. Fish Sci 78:905–910
Ueno R, Hamada-Sato N, Urano N (2003) Fermentation of molasses by several yeasts from hot spring drain and phylogeny of unique isolate producing ethanol at 55 °C. J Tokyo Univ Fish 90:23–30
Ueno R, Urano N, Kimura S (2002) Effect of temperature and cell density on ethanol fermentation by a thermotolerant aquatic yeast strain isolated from a hot spring environment. Fish Sci 68:571–578
Ueno R, Urano N, Kimura S (2001) Characterization of thermotolerant, fermentative yeasts from hot spring drainage. Fish Sci 67:138–145
Urano N, Hirai H, Ishida M, Kimura S (1998) Characterization of ethanol-producing marine yeasts isolated from coastal water. Fish Sci 64:633–637
Kumar A, Singh LK, Ghosh S (2009) Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipites. Bioresour Technol 100:3293–3297
Obara N, Enoki M, Okai M, Ueda K, Ishida M, Urano N (2014) Bioethanol production by novel yeasts with xylose fermentation activities. Stud Sci Technol 3:1–6
Miyoshi T, Uchida M, Kaneniwa M, Yoshida G (2013) Collection and component analysis of aquatic plants with a scope for fermentative utilization. J Fish Technol 6:109–124
Somogyi M (1952) Notes on sugar determination. J Biol Chem 19:195
Uchida M, Nakayama A (1993) Isolation of Laminaria-frond decomposing bacteria from Japanese coastal waters. Nippon Suisan Gakkaishi 59:1865–1871
Uchida M, Maeda T, Shiba T (2002) Phylogenic analysis of three marine bacteria that have an ability to decompose Laminaria japonica. Fish Sci 68:703–705
Matsushima R, Danno H, Uchida M, Ishihara K, Suzuki T, Kaneniwa M, Ohtsubo Y, Nagata Y, Tsuda M (2010) Analysis of extracellular alginate lyase and its gene from a marine bacterial strain, Pseudoalteromonas atlantica AR06. Appl Microbiol Biotechnol 86:567–576
Percival E (1979) Polysaccharides of green, red and brown seaweeds—their basic structure, biosynthesis and function. Br Phycol J 14:103–117
Carl C, de Nys R, Paul NA (2014) The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS One 9:e98700
Hernandez I, Peralta G, PerezLlorens JL, Vergara JJ, Niell FX (1997) Biomass and dynamics of growth of Ulva species in Palmones river estuary. J Phycol 33:764–772
Miller SM, Hurd CL, Wing SR (2011) Variations in growth, erosion, productivity, and morphology of Ecklonia radiata (Alariaceae; Laminariales) along a fjord in southern New Zealand. J Phycol 47:505–516
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takagi, T., Uchida, M., Matsushima, R. et al. Comparison of ethanol productivity among yeast strains using three different seaweeds. Fish Sci 81, 763–770 (2015). https://doi.org/10.1007/s12562-015-0875-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-015-0875-6