Abstract
Background
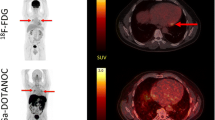
Gallium-68 Dotatate binds preferentially to somatostatin receptor (sstr) subtype-2 (sstr-2) on inflammatory cells. We aimed at investigating the potential clinical use of sstr-targeted imaging for the detection of myocardial inflammation.
Methods
Thirteen patients, with suspected cardiac sarcoidosis (CS) based on clinical history and myocardial uptake on recent fluorine-18 fluorodeoxyglucose (FDG) PET, were enrolled to undergo Dotatate PET after FDG-PET (median time 37 days [IQR 25-55]). Additionally, we investigated ex-vivo the immunohistochemistry expression of sstr-2 in 3 explanted sarcoid hearts.
Results
All FDG scans showed cardiac uptake (focal/multifocal = 6, focal on diffuse/heterogeneous = 7), and 46% (n = 6) extra-cardiac uptake (mediastinal/hilar). In comparison, Dotatate scans showed definite abnormal cardiac uptake (focal/multifocal) in 4 patients, probably abnormal (heterogenous/patchy) in 3, and negative uptake in 6 cases. Similarly, 6 patients had increased mediastinal/hilar Dotatate uptake. Overall concordance of FDG and Dotatate uptake was 54% in the heart and 100% for thoracic nodal activity. Quantitatively, FDG maximum standardized uptake value was 5.0 times [3.8-7.1] higher in the heart, but only 2.25 times [1.7-3.0; P = .019] higher in thoracic nodes relative to Dotatate. Ex-vivo, sstr-2 immunostaining was weakly seen within well-formed granulomas in all 3 examined sarcoid heart specimens with no significant staining of background myocardium or normal myocardium.
Conclusion
Our preliminary data suggest that, compared to FDG imaging, somatostatin receptor-targeted imaging may be less sensitive for the detection of myocardial inflammation, but comparable for detecting extra-cardiac inflammation.






Similar content being viewed by others
Abbreviations
- PET:
-
Positron emission tomography
- FDG:
-
Fluorodeoxyglucose
- sstr-2:
-
Somatostatin receptor (sstr) subtype-2
References
Longcope WT, Freiman DG. A study of sarcoidosis; based on a combined investigation of 160 cases including 30 autopsies from the johns hopkins hospital and massachusetts general hospital. Medicine (Baltimore) 1952;31:1–132.
Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–11.
Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest 1993;103:253–8.
Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace 2013;15:347–54.
Hiramitsu S, Morimoto S, Uemura A, Kato Y, Kimura K, Ohtsuki M, et al. National survey on status of steroid therapy for cardiac sarcoidosis in japan. Sarcoidosis Vasc Diffus Lung Dis 2005;22:210–3.
Osborne MT, Hulten EA, Murthy VL, Skali H, Taqueti VR, Dorbala S, et al. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol 2016.
Yan R, Song J, Wu Z, Guo M, Liu J, Li J, et al. Detection of myocardial metabolic abnormalities by 18f-fdg pet/ct and corresponding pathological changes in beagles with local heart irradiation. Korean J Radiol 2015;16:919–28.
Mielniczuk LM, Birnie D, Ziadi MC, deKemp RA, DaSilva JN, Burwash I, et al. Relation between right ventricular function and increased right ventricular [18f]fluorodeoxyglucose accumulation in patients with heart failure. Circ Cardiovasc Imaging 2011;4:59–66.
Folco EJ, Sheikine Y, Rocha VZ, Christen T, Shvartz E, Sukhova GK, et al. Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2-deoxy-d-glucose positron emission tomography. J Am Coll Cardiol 2011;58:603–14.
Sibille L, Chambert B, Collombier L, Kotzki PO, Boudousq V. False positive 18f-fdg pet/ct in cardiac sarcoidosis. J Mol Biol Mol Imaging 2015;2:1020.
Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, et al. Imaging of myocardial inflammation with somatostatin receptor based pet/ct—a comparison to cardiac mri. Int J Cardiol 2015;194:44–9.
Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J 2015;36:2404.
Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)ga-dotanoc pet/ct and (18)f-fdg pet/ct pilot study for detection of cardiac sarcoidosis. EJNMMI Res 2016;6:52.
Smedema JP, van Kroonenburgh MJ, Snoep G, Backes W, Gorgels AP. Images in cardiovascular medicine. Cardiac sarcoidosis in a patient with hypertrophic cardiomyopathy demonstrated by magnetic resonance imaging and single photon emission computed tomography dual-isotope scintigraphy. Circulation 2004;110:e529–31.
van Hagen PM. Somatostatin receptor expression in clinical immunology. Metabolism 1996;45:86–7.
ten Bokum AM, Hofland LJ, de Jong G, Bouma J, Melief MJ, Kwekkeboom DJ, et al. Immunohistochemical localization of somatostatin receptor sst2a in sarcoid granulomas. Eur J Clin Investig 1999;29:630–6.
Balon HR, Goldsmith SJ, Siegel BA, Silberstein EB, Krenning EP, Lang O, et al. Procedure guideline for somatostatin receptor scintigraphy with (111)in-pentetreotide. J Nucl Med 2001;42:1134–8.
Bombardieri E, Ambrosini V, Aktolun C, Baum RP, Bishof-Delaloye A, Del Vecchio S, et al. 111in-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging 2010;37:1441–8.
Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302.
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007;357:2153–65.
Deckers JW, Hare JM, Baughman KL. Complications of transvenous right ventricular endomyocardial biopsy in adult patients with cardiomyopathy: A seven-year survey of 546 consecutive diagnostic procedures in a tertiary referral center. J Am Coll Cardiol 1992;19:43–7.
Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, et al. Usefulness of fasting 18f-fdg pet in identification of cardiac sarcoidosis. J Nucl Med 2004;45:1989–98.
Greulich S, Deluigi CC, Gloekler S, Wahl A, Zurn C, Kramer U, et al. Cmr imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013;6:501–11.
Maurer AH, Burshteyn M, Adler LP, Gaughan JP, Steiner RM. Variable cardiac 18FDG patterns seen in oncologic positron emission tomography computed tomography: Importance for differentiating normal physiology from cardiac and paracardiac disease. J Thorac Imaging 2012;27:263–8.
Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during pet: A randomized controlled trial. J Nucl Cardiol 2010;17:286–91.
Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol 2011;18:926–36.
Shao D, Tian R. Glucose transporters in cardiac metabolism and hypertrophy. Compr Physiol 2015;6:331–51.
Lapa C, Reiter T, Kircher M, Schirbel A, Werner RA, Pelzer T, et al. Somatostatin receptor based pet/ct in patients with the suspicion of cardiac sarcoidosis: An initial comparison to cardiac mri. Oncotarget 2016;7:77807–14.
Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail 2018;5:249–61.
Nobashi T, Nakamoto Y, Kubo T, Ishimori T, Handa T, Tanizawa K, et al. The utility of pet/ct with (68)ga-dotatoc in sarcoidosis: Comparison with (67)ga-scintigraphy. Ann Nucl Med 2016;30:544–52.
Sharma S, Singh AD, Sharma SK, Tripathi M, Das CJ, Kumar R. Gallium-68 dota-noc pet/ct as an alternate predictor of disease activity in sarcoidosis. Nucl Med Commun 2018;39:768–78.
Acknowledgments
This work was supported by a grant from the Radiological Society of North America Research & Education Foundation Board of Trustees (RF1632), Institutional Funds, and a Training Grant from the National Institutes of Health (1T32HL094301).
Disclosure
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bravo, P.E., Bajaj, N., Padera, R.F. et al. Feasibility of somatostatin receptor-targeted imaging for detection of myocardial inflammation: A pilot study. J. Nucl. Cardiol. 28, 1089–1099 (2021). https://doi.org/10.1007/s12350-019-01782-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01782-0