Abstract
Introduction
Reducing intraocular pressure (IOP), the only modifiable risk factor for open-angle glaucoma (OAG), is important for the preservation of vision and slowing of disease progression. Preservative-free tafluprost (0.0015%)/timolol (0.5%) fixed combination (PF Taf-T FC) is an approved combination therapy for OAG treatment. The VISIONARY study aimed to evaluate the effectiveness and tolerability of PF Taf-T FC in real-world clinical settings. Here, we present the results from the United Kingdom (UK) and Ireland.
Methods
This observational, multicentre, European, prospective study recorded data during routine clinic appointments on the use of PF Taf-T FC for the treatment of OAG and ocular hypertension (OHT) in patients whose disease was insufficiently controlled on a prostaglandin analogue (PGA) or beta blocker monotherapy or who did not tolerate these medications. Mean change in IOP, symptom severity, changes in clinical signs, and tolerability were investigated over 6 months.
Results
Eighty-two patients were recruited in the UK and Ireland. After 6 months of PF Taf-T FC treatment, mean IOP was significantly reduced from 22.0 to 16.2 mmHg in the UK group and from 18.6 to 14.1 mmHg in the Ireland group. In the UK (65 patients), 49 adverse events (AEs) were reported, of which 3 were serious. No AEs were reported in the Ireland group (17 patients). Overall, 91.9% of UK physicians reported PF Taf-T FC treatment to be the same or better than prior medication for improving clinical signs; 90.0% of UK patients reported PF Taf-T FC treatment to have good or very good tolerability.
Conclusions
Treatment with PF Taf-T FC resulted in significant reductions in mean IOP over 6 months. Patients and physicians reported that treatment was well tolerated. These data demonstrate real-world efficacy of PF Taf-T FC for the treatment of OAG and OHT in routine clinical practice in the UK and Ireland.
Trial registration
European Union electronic Register of Post-Authorisation Studies (EU PAS) register number, EUPAS22204.
Similar content being viewed by others
Why carry out this study? |
Preservative-free tafluprost (0.0015%)/timolol (0.5%) fixed combination (PF Taf-T FC) is a combination therapy for the treatment of open-angle glaucoma (OAG). |
PF Taf-T FC has been shown to reduce intraocular pressure (IOP) in patients with OAG in randomised clinical trials. |
This study investigated the effectiveness and tolerability of PF Taf-T FC in real-world clinical settings in Europe. This paper focuses on results from the United Kingdom (UK) and Ireland, where previously prescribed monotherapy is most likely to be prostaglandin analogues. |
What was learned from the study? |
Country-specific analysis found that, over 6 months, PF Taf-T FC treatment significantly reduced mean IOP in patients with OAG in the UK and Ireland. |
This study demonstrates real-world efficacy and tolerability of PF Taf-T FC treatment in routine clinical practice in these countries. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14261039.
Introduction
Increased intraocular pressure (IOP) is an important risk factor for the development of open-angle glaucoma (OAG). Reducing IOP is the only available mechanism for slowing disease progression and is critical for preserving vision and halting visual field loss [1].
Topical IOP-lowering monotherapy medications are first-line treatments for OAG [1,2,3,4]. In England, the National Institute for Health and Care Excellence (NICE) recommend prostaglandin analogues (PGA) [3] while other European countries recommend beta blockers [4]. However, if these do not result in a reduction to target IOP, additional therapy is required [4]. Commonly, this is a combination of a PGA and a beta blocker. Fixed-dose combinations of PGAs and beta blockers are often used as they minimise drop requirement and improve adherence [4, 5]. Preservative-free therapies are increasingly being adopted as they are associated with a lower risk of ocular surface disease (OSD) [6, 7] than preserved drops, which further promotes adherence. Preservative-free tafluprost (0.0015%)/timolol (0.5%) fixed combination (PF Taf-T FC) is a preservative-free PGA/beta blocker fixed combination treatment approved for the treatment of OAG [8]. Previous clinical trials have shown a low rate of hyperaemia with PF Taf-T FC treatment [8, 9].
Randomised controlled trials (RCTs) are the gold standard for the collection of robust safety and efficacy data. However, they are unable to represent the full spectrum of real-world scenarios [10]. Real-world evidence from observational studies recording data from standard clinical practice can complement the rigorous safety and efficacy data produced by RCTs by comparing the results of RCTs with those pragmatically collected through standard clinical practice [11].
The VISIONARY study evaluated the effectiveness and tolerability of PF Taf-T FC treatment in patients with OAG or ocular hypertension (OHT) over 6 months who previously had an insufficient response to either PGA or beta blocker monotherapy, or who did not tolerate these medications, and were judged by their physician to benefit from preservative-free eye drops. Results from the full data set have previously been published [12]. This paper presents the data and further country-specific analysis for patients in the UK and Ireland, and discusses the potential differences between these countries and the full data set. The UK cohort was chosen to be included in this country-specific analysis as, unlike most European countries, UK guidelines recommend PGAs rather than beta blockers as first-line treatments for OAG. Differences in the methodology to measure mean IOP in Ireland (Goldmann vs iCARE tonometry methods), according to standard clinical practice in the country, mean that detailed analysis may allow exploration of the differences in IOP change that may be experienced by patients in real-world practice.
Methods
Patients
The inclusion and exclusion criteria are summarised in Supplementary Table S1. Details of the trial design are published elsewhere [12]. In brief, male or female patients aged at least 18 years with a diagnosis of OAG or OHT were recruited. Patients with insufficient IOP control on, or intolerance to, monotherapy with topical beta blockers or PGAs, and who were judged by their physician to benefit from preservative-free eye drops, were recruited to the study. All eligible patients attending participating clinics were invited to participate in the study. Patients were required to provide informed consent before enrolling.
Study Design
In line with European Medicines Agency (EMA) requirements, the study was registered under the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP®) European Union electronic Register of Post-Authorisation Studies (EU PAS Register) (EU PAS register number EUPAS22204). The study complied with the principles of the Declaration of Helsinki. The study protocol was approved by the institutional review board (IRB) or independent ethics committee (IEC) at each centre/institution. The study centres/institutions are listed in the Acknowledgments alongside the relevant principal investigator.
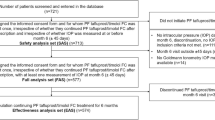
A 6-month, observational, multicentre, prospective study was undertaken (Fig. 1). Eleven countries were included in the study; however, this paper presents results from the UK and Ireland only [12]. Data were recorded during routine clinical visits and were entered into electronic case report forms. Patients were prescribed PF Taf-T FC treatment as one drop daily [12]. Baseline data were recorded within 7 days of PF Taf-T FC treatment initiation. Follow-up clinical visits were completed after 4 weeks (± 7 days), 12 weeks (± 7 days) and 6 months (± 45 days) of PF Taf-T FC treatment. However, data collection at 4 and 12 weeks was optional.
Study Procedures and Evaluations
The primary endpoint of the study was mean absolute IOP change (mmHg) from baseline to 6 months after PF Taf-T FC treatment initiation. Secondary endpoints were mean absolute IOP change from baseline at interim visits (weeks 4 and 12) and patient responder rate, defined as the percentage of patients with an IOP change from baseline of at least 20% at week 12. Changes in ocular signs and severity of symptoms, including conjunctival hyperaemia and visual acuity, at week 4, week 12 and month 6 were investigated. Additionally, change in subjective symptoms with PF Taf-T FC treatment were explored, including differences in severity. Effectiveness and patient compliance as assessed by the physician, and tolerability as assessed by the patient, of PF Taf-T FC were also investigated.
Demographic, diagnosis and previous treatment data were recorded at baseline (Table 1). In addition, a baseline IOP measurement was recorded, including the time of day at which the measurement took place. IOP was measured by Goldmann applanation tonometry in the UK, as per routine clinical practice recommended by NICE guidance [3]. At the study enrolment visit in Ireland, IOP was measured using iCARE applanation tonometry for most patients; however, for some patients Goldmann tonometry was used. As outlined in local clinical standard guidelines, at the study clinic in Ireland, standard practice was to use iCARE tonometry for follow-up tests, with results being confirmed with Goldmann tonometry if IOP readings were less than 10 mmHg or over 20 mmHg. In the overall study analysis, assessments using the iCARE method were excluded [12]; however, in these country-specific analyses they were included as a separate group to ensure patients were as reflective of the Irish patient population as possible. For all patients, the same method of IOP measurement was used at baseline and month 6. The clinical signs and subjective symptoms of glaucoma were also recorded at baseline (Table 1).
During the follow-up visits, the duration of PF Taf-T FC treatment was recorded. Conjunctival hyperaemia and corneal fluorescein staining (CFS) assessments were mandatory at month 6, and optional at weeks 4 and 12. Additional data were reported by physicians and patients. Physician evaluations included the effectiveness and clinical outcomes of PF Taf-T FC treatment, as well as patient compliance (judged as better, the same, or worse than prior medication). Patients assessed the tolerability of PF Taf-T FC treatment (very good, good, satisfactory or poor), reasons for discontinuation of treatment if applicable, and any adverse events (AEs) occurring throughout the study.
Statistical Analysis
For patients who had both eyes treated, the eye with the higher IOP reading at baseline was designated the index eye and included in the analysis. If both eyes had an equal IOP reading, the right eye was included. Analysis was completed on the full UK and Ireland data set, which included any patients who provided informed consent, were prescribed PF Taf-T FC at least once and had at least one IOP measurement at 6 months (± 45 days) after treatment initiation. Analysis of any specific endpoint included patients with complete data on the variables required. Patients were included in the follow-up period regardless of whether they had discontinued PF Taf-T FC, with the aim of reflecting the real-life treatment environment. The primary endpoint was assessed for both countries, regardless of the number of patients included; however, secondary endpoints were only investigated if the study population for the country was at least 20 patients. Normality tests were carried out and, if the results were normally distributed, a paired t test was conducted to assess statistical significance. If the results were not normally distributed, a Wilcoxon signed rank test was conducted.
AEs were summarised using system organ class (SOC) terms and preferred terms (PTs) [13]. Severity, causality, relatedness to PF Taf-T FC and outcomes were also recorded.
Results
Patient Disposition and Baseline Characteristics
The proportion of patients included and excluded from the full analysis set overall, across all countries included in the VISIONARY study, has been previously reported [12]. In total, 65 patients were enrolled from the UK and 17 patients were enrolled from Ireland (Table 2). As the Ireland group included fewer than 20 patients, primary endpoints were analysed for both the UK and Ireland, but secondary endpoints were only analysed for the UK, in accordance with the statistical analysis plan (SAP). The baseline characteristics are summarised in Table 2. At baseline, the mean (standard deviation [SD]) IOP was 22.0 (4.5) mmHg for the UK patient group. In Ireland, patients who had their IOP measured using the Goldmann method had a mean (SD) measurement of 17.0 (2.7) mmHg; for patients in the iCARE method group, the mean (SD) IOP was 18.6 (5.1) mmHg.
Mean IOP Reduced with PF Taf-T FC Treatment
PF Taf-T FC treatment significantly reduced mean (SD) IOP at month 6 in patients from the UK, from 22.0 (4.5) mmHg at baseline to 16.2 (2.9) mmHg at month 6 (p < 0.0001; Fig. 2a). In patients in the Ireland group, mean (SD) IOP was significantly reduced at month 6 compared to baseline for the iCARE subgroup (n = 14; baseline, 18.6 [5.1] mmHg; month 6, 14.1 [3.0] mmHg; p = 0.003; Fig. 2b). For patients in the Goldmann subgroup (n = 3) mean IOP reduced from 17.0 (2.7) mmHg at baseline to 13.3 (1.2) mmHg after 6 months of PF Taf-T FC treatment; however, the number of patients in this category was too small to meaningfully assess statistical significance (Fig. 2b). In the UK group, significant reductions (p < 0.0001) were also seen at weeks 4 and 12 compared to baseline (baseline [n = 65], 22.0 [4.5] mmHg; week 4 [n = 52], 17.2 [3.6] mmHg; week 12 [n = 52], 16.6 [3.3] mmHg; Fig. 2a). For the UK patient group, the percentage of responders was 61.5% at 6 months (Fig. 2a).
Mean IOP change in the UK and Ireland patient groups over time. a Mean IOP change and responder rates over time in the UK patient group; b mean IOP change, split by method of IOP measurement, in the Ireland patient group. *p < 0.01; **p < 0.0001 using two-sided paired t test. Responders are patients with change in IOP of ≥ 20% from baseline. Error bars indicate SD. IOP intraocular pressure, SD standard deviation
Change in CFS Score in UK Patients Over Time
For patients in the UK group, CFS score change over time was analysed. Most UK patients had a CFS score of 0 throughout the study (Fig. 3). The percentage of patients with a CFS score of 0 ranged from 52.5% to 60.9% across time points. Throughout the study, all patients had a score of III or less, except for one patient who had a score of IV at month 6. Improvements in CFS scores at week 4, week 12 or month 6 compared with baseline were not statistically significant using a Bhapkar test (week 4, p = 0.51; week 12, p = 0.18; month 6, p = 0.28).
Change in Conjunctival Hyperaemia Severity in UK Patients Over Time
Most UK patients had mild conjunctival hyperaemia at baseline (Fig. 4). The percentage of patients with mild conjunctival hyperaemia decreased from 61.3% at baseline to 49.2% at month 6; this was reflected in an increase in patients with no conjunctival hyperaemia (19.4% of patients at baseline, compared to 35.4% of patients at month 6). However, improvements in conjunctival hyperaemia grade at week 4, week 12 or month 6 compared to baseline were not statistically significant using a Bhapkar test (week 4, p = 0.32; week 12, p = 0.10; month 6, p = 0.06).
Other Clinical Signs in UK Patients Over Time
In the UK, best corrected visual acuity (BCVA) data were available for 62 patients at baseline with a median decimal score of 0.7 (interquartile range [IQR] 0.3). BCVA data were available for 49 patients at week 4 (median 1.0; IQR 0.3), 52 patients at week 12 (median 0.9; IQR 0.3) and 62 patients at month 6 (median 0.8; IQR 0.3). The change in BCVA decimal score from baseline to any time point was not statistically significant (p ≥ 0.35 for all time points). For Schirmer’s test, no data were recorded at baseline in the UK; therefore, no change from baseline data could be calculated. Tear break-up time (TBUT [seconds]) was recorded for 41 patients at baseline (median 5.0; IQR 7.0), 38 patients at week 4 (median 9.0; IQR 5.0), 41 patients at week 12 (median 6.0; IQR 5.0) and 47 patients at month 6 (median 6.0; IQR 5.0). For the 33 patients for whom TBUT data were available at baseline and week 4, a change of 4.0 s from baseline was statistically significant (p = 0.001); changes at week 12 and month 6 were not statistically significant compared to baseline (p ≥ 0.14).
Subjective Symptoms in UK Patients at Month 6
Analysis of the subjective symptoms recorded in the UK found that, of those who answered, 100% of physicians reported PF Taf-T FC treatment to be the same or better than prior medication for IOP control after 6 months of treatment (Table 3). Similarly, 91.9% of physicians rated PF Taf-T FC treatment as the same or better than prior medication for improving clinical signs, and 100% of physicians rated the treatment as the same or better than prior medication for patient compliance after 6 months of treatment (Table 3). At month 6, of the 60 patients who answered, 90.0% reported PF Taf-T FC treatment to have good or very good tolerability (Table 3).
Safety Outcomes
In the UK group, 49 AEs were recorded (Table 4; Supplementary Table S2). Of these, 31 (63.3%) were mild, 13 (26.5%) were moderate, and 5 (10.2%) were severe. There were three (6.1%) serious AEs reported; one case of retinal vein occlusion, one case of renal impairment and one case of cerebrovascular accident. There were no fatal AEs. As assessed by the investigator, 26 (53.1%) were related to PF Taf-T FC treatment. The most common AEs were classified as eye disorders (34 events; 69.4% of AEs), including eye pain (14; 28.6%) and dry eye (3; 6.1%). There were no AEs reported in the Ireland group.
Discussion
PF Taf-T FC treatment resulted in a significant reduction in mean IOP at month 6 in both the UK and Ireland (iCARE) patient populations, supporting its effectiveness as a treatment for OAG and OHT. This reduction was seen from week 4 in the UK patient population (p < 0.0001; 51.9% patients showed more than 20% reduction in mean IOP). There was no statistical difference in the other clinical symptoms measured. Physicians reported that PF Taf-T FC treatment was effective, and patients reported that it showed good tolerability. Safety data indicate that PF Taf-T FC treatment was well tolerated, with few serious AEs.
In the Ireland patient group, IOP was measured by two different methods, which have been found to produce comparable results for patients with low to moderate IOP [14, 15]. Reductions in IOP measured using the iCARE method were statistically significant (p < 0.01). In Ireland both the iCARE and Goldmann methods are used and by including both methods in these analyses, this study better reflects real-world practice in Ireland.
Compared with the overall results from this study [12], patients in the UK and Ireland were more likely to be switching from PGA therapy, in line with NICE treatment guidelines [3]. In the full study population, the percentage of patients switching from PGA range from 39.2% in Latvia, to 90.8% in the UK and 94.1% in Ireland [12]. PF Taf-T FC treatment has been found to result in a smaller reduction in mean IOP in patients switching from PGA therapy, compared with those switching from beta blockers [12]. However, in this country-specific analysis, reductions in mean IOP are clinically and statistically significant despite the smaller sample size and more than 90% of patients switching from PGA therapy, demonstrating that reductions with PF Taf-T FC treatment are likely to be found regardless of previous treatment.
The analysis of the overall results also demonstrated a statistically significant reduction in CFS score and conjunctival hyperaemia with PF Taf-T FC treatment [12]. This statistical significance was not seen in these country-specific treatment groups; however, there was a trend towards a reduction in both likely reflecting the smaller numbers in these patient groups. This may also be due to the difference in previous monotherapies between the UK and Ireland and the other countries in this study. Patient numbers in the CFS and conjunctival hyperaemia analyses were lower at weeks 4 and 12 than month 6. This is likely to be due to the recording of these data being optional at these interim follow-up visits. Although these data do not demonstrate significant improvements in CFS and conjunctival hyperaemia, they also do not suggest that these clinical symptoms worsen with combination therapy. An improvement in CFS would be expected when patients switch to a preservative-free therapy; however, data assessing the number of patients previously taking preservative-free treatment were not recorded, so it was not possible to investigate this change.
This study demonstrates PF Taf-T FC effectiveness in routine clinical practice, supporting safety and efficacy data from RCTs [9, 16, 17]. Patients included in RCTs often do not represent the full spectrum of patients diagnosed with a disease, as patients with comorbidities are often excluded because they do not meet the strict inclusion criteria of such trials [18]. Additionally, non-pragmatic RCTs do not accurately reflect variations in routine clinical practice where it may be necessary to balance perceived ideal treatments with practical considerations and ensure the best quality of life for patients is achieved. In the present study, however, no washout period between previous treatment and PF Taf-T FC treatment initiation and a wider range of patients included than in clinical trials (e.g. patients with ocular surface abnormalities) are examples of how these data are more reflective of routine clinical practice than previous clinical trials investigating PR Taf-T FC efficacy. This study provides ophthalmologists with data that may be more reflective of real-world treatment with PF Taf-T FC, particularly in UK and Ireland. Real-world data on time to response, the percentage of responders and the range of glaucoma diagnoses for which PF Taf-T FC treatment is effective may help guide treatment decisions in the clinic.
Limitations
The sample size, particularly in the Ireland group, is small; therefore these results may not be generalisable to wider patient populations in the UK and Ireland. Additionally, since this study investigated adult patients with OAG and OHT, the results are not applicable to angle-closure glaucoma and childhood glaucomas. In the Ireland group, the inclusion of both Goldmann and iCARE subgroups adds some noise to the study analysis. However, the presence of both patient groups is reflective of real-world clinical practice in Ireland, where there are no guidelines that recommend the use of Goldmann IOP measurement methods in all patients with glaucoma. The study did not take into account the reported disparities between the two instruments that were used for measuring IOP among patients in the study, iCARE applanation tonometry and Goldmann tonometry [14, 15], though the same method of IOP measurement was used at baseline and month 6 for all patients. In-depth details relating to the methods of IOP measurement were lacking. For example, no information was recorded to understand whether the same physician measured and recorded IOP and if these measurements were taken at the same time of day. It was also unclear if only one reading was taken and reported, or if several IOP readings were taken and the average value reported. Patients in this study had only previously been treated with monotherapies; improvements may not be the same if switching from a different combination therapy to PF Taf-T FC treatment. Further, data regarding whether patients were previously on preservative-free treatment, how long patients had been on monotherapy prior to baseline or which medications were used by patients after discontinuation of PF Taf-T FC were not recorded, which limited the exploration of these variables.
Conclusion
In patients, in the UK and Ireland, with OAG and OHT that was insufficiently controlled by monotherapies, PF Taf-T FC treatment demonstrated real-life efficacy in routine clinical practice. A statistically significant reduction in mean IOP was shown from 4 weeks of treatment. This reduction was maintained up to 6 months. In addition, safety data and physician- and patient-reported data demonstrate PF Taf-T FC treatment is well tolerated.
Change history
30 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12325-021-01988-0
References
International Council of Ophthalmology. Guidelines for glaucoma care. 2016. http://www.icoph.org/downloads/ICOGlaucomaGuidelines.pdf. Last Accessed 13 May 2020.
Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma Preferred Practice Pattern(®) guidelines. Ophthalmology. 2016;123(1):P41-p111.
Glaucoma: diagnosis and management. NICE guideline. 2017. https://www.nice.org.uk/guidance/ng81/resources/glaucoma-diagnosis-and-management-pdf-1837689655237. Last Accessed 22 Sept 2020.
European Glaucoma Society. Terminology and guidelines for glaucoma. 2014. https://www.eugs.org/eng/egs_guidelines_download.asp. Last Accessed 13 May 2020.
Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–9.
Holló G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15(12):1737–47.
Mohammed I, Kulkarni B, Faraj LA, Abbas A, Dua HS, King AJ. Profiling ocular surface responses to preserved and non-preserved topical glaucoma medications: a two-year randomised evaluation study. Clin Exp Ophthalmol. 2020;48(7):973–82.
Konstas AG, Katsanos A, Athanasopoulos GP, et al. Preservative-free tafluprost/timolol fixed combination: comparative 24-h efficacy administered morning or evening in open-angle glaucoma patients. Expert Opin Pharmacother. 2018;19(18):1981–8.
Pfeiffer N, Traverso CE, Lorenz K, et al. A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components. Adv Ther. 2014;31(12):1228–46.
Spitzer E, Cannon CP, Serruys PW. Should real-world evidence be incorporated into regulatory approvals? Expert Opin Drug Saf. 2018;17(12):1155–9.
Bartlett VL, Dhruva SS, Shah ND, Ryan P, Ross JS. Feasibility of using real-world data to replicate clinical trial evidence. JAMA Netw Open. 2019;2(10):e1912869-e.
Oddone F, Tanga L, Kóthy P, Holló G. Treatment of open-angle glaucoma and ocular hypertension with preservative-free tafluprost/timolol fixed-dose combination therapy: the VISIONARY study. Adv Ther. 2020;37(4):1436–51.
World Health Organization. 2011. Introductory guide for MedDRA. https://www.who.int/medical_devices/innovation/MedDRAintroguide_version14_0_March2011.pdf. Last Accessed 04 Jan 2021.
Gao F, Liu X, Zhao Q, Pan Y. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer. Exp Ther Med. 2017;13(5):1912–6.
Chen M, Zhang L, Xu J, et al. Comparability of three intraocular pressure measurement: iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. 2019;19(1):225.
Holló G, Hommer A, Antón López A, Ropo A. Efficacy, safety, and tolerability of preservative-free fixed combination of tafluprost 0.0015%/timolol 0.5% versus concomitant use of the ingredients. J Ocul Pharmacol Ther. 2014;30(6):468–75.
Holló G, Katsanos A. Safety and tolerability of the tafluprost/timolol fixed combination for the treatment of glaucoma. Expert Opin Drug Saf. 2015;14(4):609–17.
Averitt AJ, Weng C, Ryan P, Perotte A. Translating evidence into practice: eligibility criteria fail to eliminate clinically significant differences between real-world and study populations. NPJ Digit Med. 2020;3:67.
Acknowledgements
The authors thank the patients, the investigators and their teams who took part in this study.
Funding
This study was sponsored by Santen SA. This article was based on the original VISIONARY study (EUPAS22204) and was sponsored by Santen UK Ltd. The Rapid Service and Open Access Fees were funded by Santen UK. Support for third-party writing assistance for this article, provided by Emma Phillips, PhD, Costello Medical, UK, was funded by Santen UK Ltd in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Medical writing funded by Santen UK Ltd. All authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing/Editorial Assistance
The authors also acknowledge Fiona Thompson, BA (Hons), St Albans, UK, for publication coordination and Layla Ndiaye, BSc, Emma Phillips, PhD, and Kerris Chappell-Smith, BA, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Substantial contributions to study conception and design: Ejaz Ansari, Jasna Pavicic-Astalos, Filis Ayan, Anthony J. King, Matthew Kinsella, Eugene Ng, Anca Nita; substantial contributions to analysis or interpretation of the data: Ejaz Ansari, Jasna Pavicic-Astalos, Filis Ayan, Anthony J. King, Matthew Kinsella, Eugene Ng, Anca Nita; drafting the article or revising it critically for important intellectual content: Ejaz Ansari, Jasna Pavicic-Astalos, Filis Ayan, Anthony J. King, Matthew Kinsella, Eugene Ng, Anca Nita; final approval of the version of the article to be published: Ejaz Ansari, Jasna Pavicic-Astalos, Filis Ayan, Anthony J. King, Matthew Kinsella, Eugene Ng, Anca Nita.
List of Investigators
In particular, the authors thank the following individuals for contributing to the study; D. Broadway, Spire Norwich Hospital, Norwich, UK; K. Claridge, Royal Cornwall Hospital, Truro, Cornwall, UK; J. Kirwan, Queen Alexandra Hospital, Portsmouth Hospitals NHS Trust, Portsmouth, Hampshire, UK; A. Moosavi, Milton Keynes University Hospital NHS Foundation Trust, Milton Keynes, UK; S. Ruben, Southend University Hospital NHS Foundation Trust, Westcliff-On-Sea, Essex, UK; M. Smith, Royal Devon & Exeter NHS Foundation Trust, Exeter, Devon, UK. The authors acknowledge ICON for clinical trial management and statistical analysis.
Disclosures
Jasna Pavicic-Astalos: Research contracts from Santen Pharmaceuticals and Glaukos, research contracts and educational grants from Allergan and Thea Pharmaceuticals. Filis Ayan: Employee of Santen UK Ltd. Anthony J. King: Educational lecture fees received from Thea Pharmaceuticals and Allergan. Advisory board member for Allergan and Thea Pharmaceuticals. Eugene Ng: Research contract and / or consulting fees from Alcon, Novartis, Bayer, Carl Zeiss Meditec, Allergan, Santen, IVERIC bio, Alimera Sciences, SIFI Medtech, IMS Health, Medisoft, Roche, OD-OS. Ejaz Ansari, Matthew Kinsella and Anca Nita have nothing to disclose.
Compliance with Ethics Guidelines
The study complied with the principles of the Declaration of Helsinki. The study protocol was approved by the institutional review board (IRB) or independent ethics committee (IEC) at each centre/institution listed above. All patients were required to provide informed consent before enrolling.
Data Availability
The datasets used during and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
The original online version of this article was revised due to update in article text.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ansari, E., Pavicic-Astalos, J., Ayan, F. et al. Treatment of Open-Angle Glaucoma and Ocular Hypertension with Preservative-Free Tafluprost/Timolol Fixed-Dose Combination Therapy: UK and Ireland Results from the VISIONARY Study. Adv Ther 38, 2990–3002 (2021). https://doi.org/10.1007/s12325-021-01725-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01725-7