Abstract
Purpose
To model the American College of Rheumatology (ACR) outcomes, cost-effectiveness, and budget impact of certolizumab pegol (CZP) (with and without a hypothetical risk-sharing scheme at treatment initiation for biologic-naïve patients) versus the current mix of reimbursed biologics for treatment of moderate-to-severe rheumatoid arthritis (RA) in Finland.
Methods
A probabilistic model with 12-week cycles and a societal approach was developed for the years 2015–2019, accounting for differences in ACR responses (meta-analysis), mortality, and persistence. The risk-sharing scheme included a treatment switch and refund of the costs associated with CZP acquisition if patients failed to achieve ACR20 response at week 12. For the current treatment mix, ACR20 at week 24 determined treatment continuation. Quality-adjusted life years were derived on the basis of the Health Utilities Index.
Results
In the Finnish target population, CZP treatment with a risk-sharing scheme led to a estimated annual net expenditure decrease ranging from 1.7% in 2015 to 5.6% in 2019 compared with the current treatment mix. Per patient over the 5 years, CZP risk sharing was estimated to decrease the time without ACR response by 5%-units, decrease work absenteeism by 24 days, and increase the time with ACR20, ACR50, and ACR70 responses by 5%-, 6%-, and 1%-units, respectively, with a gain of 0.03 quality-adjusted life years. The modeled risk-sharing scheme showed reduced costs of €7866 per patient, with a more than 95% probability of cost-effectiveness when compared with the current treatment mix.
Conclusion
The present analysis estimated that CZP, with or without the risk-sharing scheme, is a cost-effective alternative treatment for RA patients in Finland. The surplus provided by the CZP risk-sharing scheme could fund treatment for 6% more Finnish RA patients.
Funding
UCB Pharma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most European nations attempt to limit pharmaceutical expenditure. Popular cost containment strategies include external and internal reference pricing, positive and negative reimbursement lists, generic substitution, price freezes and cuts, and mandatory health technology assessment [1, 2]. Introduction of new treatment options, an increasing health and social care cost burden to society, and a lack of economic growth are major challenges for the finance and sustainability of health care globally. However, access to innovative treatments and sufficient compensation for innovation should be ensured if medical need and sufficient efficacy are observed.
To overcome these challenges, institutional payment arrangements, including risk-sharing schemes (RSSs) [3], have been proposed and implemented. RSSs focused on treatment benefit and efficiency have been referred to as “performance-based contracts,” “efficiency stipulation schemes,” or “effectiveness guarantee schemes” [4]. However, from our review of the PubMed indexed literature, implementation and health economic (HE) analyses of the potential impact of efficiency-oriented RSSs are rare. Instead, financial agreements (e.g., patient access schemes) in which the patient receives the drug at a discounted cost or free of charge for a predefined period [1], appear to be more commonly implemented and evaluated.
Rheumatoid arthritis (RA) is a debilitating, chronic autoimmune disorder, causing significant economic and human burden [5–9]. The prevalence of RA in Finland has been estimated at around 0.8% of the population [10]. RA cannot be cured [11], and the real-world effectiveness of currently available treatments may not be optimal (i.e., not all patients achieve remission) for most Finnish patients who have moderate-to-severe RA [12]. The objective of this study was to model the HE impact of a hypothetical, efficiency-oriented early RSS based on current drug labeling guidelines in the treatment of moderate-to-severe RA in Finland.
Methods
A probabilistic model was developed to evaluate the 5-year HE impact (cost-effectiveness and budget impact) of certolizumab pegol (CZP) with and without an RSS at treatment initiation as an alternative biologic option for biologic-naïve patients with moderate-to-severe RA (moderate RA, 3.2 < DAS28 ≤ 5.1; severe RA, DAS28 > 5.1) compared with the treatment mix of reimbursed biologic disease-modifying antirheumatic drugs (bDMARDs; abatacept, adalimumab, CZP, etanercept, golimumab, infliximab, tocilizumab) currently used in Finland. The use of a treatment mix as a base case comparator for the CZP RSS was based on the following rationale: (1) a CZP RSS would be more likely to complement a mix of treatments over time, rather than a single treatment; (2) inclusion of both incident and prevalent RA patients necessitates a mix; (3) budget impact estimates are more relevant for a mix, and (4) decision makers often consider the comparator to be “current care” and not a single treatment. However, sensitivity analyses report results of CZP RSS versus single treatment scenarios.
RSSs or patient access schemes for publicly reimbursed pharmaceuticals were not part of Finnish reimbursement practice at the time of analysis [13–15]. However, since January 1, 2017, the Finnish Pharmaceuticals Pricing Board has considered RSSs proposed as part of new reimbursement applications on a drug-by-drug basis. The general framework is an agreement-based conditional reimbursement that needs to be separately applied [16, 17] and that can include an RSS. This study models a potential—yet hypothetical—RSS for the Finnish situation. We are not aware of any other formal analyses that explore the option to include an RSS as part of a Finnish reimbursement agreement.
Outcomes
Estimated outcomes included per-patient incremental cost-effectiveness and net budget impact at the target population level. Secondary estimated outcomes included per-patient survival (life years), quality-adjusted survival (quality-adjusted life years, QALYs), and lost productivity (absenteeism in terms of work days lost, valued by the human-capital approach, HCA [18]) over 5 years. Treatment costs were estimated at the patient and target population levels. Although lifetime modeling is often considered in modeled HE evaluations, 5-year modeling was used in this study on the basis of clinical and economic rationales, including changes in treatment recommendations, improved care practices, market fluctuations (changes in the market shares of drugs, biosimilars, and new treatments), and potential advances in our understanding of RA within 5–10 years. In addition, a 5-year timeframe is associated with significantly less extrapolation uncertainty than a lifetime horizon, and follow-up data related to disease progression in Finnish RA patients treated with bDMARDs for more than 5 years are lacking.
A cost-effectiveness acceptability frontier was drawn to determine optimal treatments, in terms of net monetary benefit, at different willingness-to-pay levels. The commonly referenced willingness-to-pay values include €30,000 and €50,000 per QALY gained in Finland [19–24]. However, Finland does not have an official threshold for cost-effectiveness [15, 25]. Threshold estimates for the UK could potentially be applicable in Finland: most plausible threshold, around €25,000 per QALY gained, plausible threshold, around €37,000 per QALY gained [26], or end-of-life threshold, around €55,000 [27] per QALY gained, on the basis of population-weighted decisions. The Finnish Medicines Agency considered €68,000 per QALY gained to approach the maximum cost-effectiveness threshold for a life-threatening disease [28], which concurs with earlier Finnish average opinion [29]. Because RA is not a life-threatening disease or end-of-life condition, incremental cost-effectiveness ratios exceeding €37,000 per QALY gained are probably not acceptable for RA in Finland, or at least necessitate additional evidence.
Clinical response, assessed by American College of Rheumatology (ACR) response criteria, and population-level budget impact were estimated without discounting [30], whereas a 3% annual rate [31] was applied to the remaining outcome measures. ACR response was selected as the primary surrogate outcome because it is the most widely reported outcome in RA trials (see Appendix A in the electronic supplementary material). ACR response was also included in the Finnish register of biologic treatments and the FIN-RACo trial.
Setting
CZP is one of the most recent tumor necrosis factor inhibitors to be licensed for the treatment of RA. Studies have shown that an early response to CZP treatment at week 12 can predict the likelihood of long-term response [32–34] (see Appendix A in the electronic supplementary material). This suggests that an RSS based on a 12-week assessment of efficacy would be feasible for CZP.
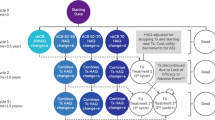
The impact of a hypothetical RSS based on the achievement of ACR20 response at week 12 was estimated. Under the RSS, biologic-naïve patients achieving ACR20 response would continue with CZP treatment, whereas failure to respond would lead to a treatment switch and subsequent refund of the costs associated with CZP acquisition. This scenario is in line with the current European League Against Rheumatism (EULAR) [35] and Finnish [36] treatment guidelines, which emphasize a treat-to-target approach in RA and the consideration of alternative treatment in the instance of early nonresponse.
The Summary of Product Characteristics (SPCs) for some first-line RA bDMARDs (e.g., abatacept, golimumab, and infliximab) do not encourage a change in treatment if response is not achieved by week 12, but rather advocate continuation of treatment, with assessment of clinical response at later time points (e.g., 24 weeks). Therefore, the RSS was applied only to CZP-treated patients. In the model base case, the continuation or switching decision for the current treatment mix was based on attainment of ACR20/50/70 response at week 24 (at minimum, ACR20 response was required for continuation).
Health Economic Modeling
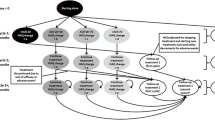
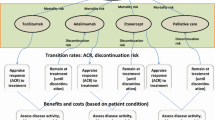
The treatment of moderate-to-severe RA after inadequate response to a conventional disease-modifying antirheumatic drug (cDMARD) was modeled. This model was structured as a fully probabilistic (including standard errors/deviations where available), open-cohort model with a 12-week cycle length, implemented in Microsoft Excel with Visual Basic for Applications (Fig. 1). Normal distributions were used to propagate the uncertainty of the following inputs: initial Health Assessment Questionnaire Disability Index (HAQ-DI) score; prediction of HAQ-DI score based on ACR response; multivariate prediction of quality of life (QoL) based on HAQ-DI score, or HAQ-DI bands-based prediction of QoL (sensitivity analysis); background mortality based on age and sex; and elevated mortality, hospitalizations, and work days lost based on HAQ-DI score. For ACR responses, logistic distributions were implemented.
CZP as the first-line bDMARD for all biologic-naïve patients (2015 onwards) was compared with a current mix of bDMARDs, with and without an RSS. The current mix of bDMARDs was assumed to consist of subcutaneously administered, reimbursed bDMARDs, and was based on the market shares of available drugs (subcutaneously administered abatacept 5.1%, adalimumab 46.9%, CZP 3.7%, etanercept 31.2%, golimumab 13.1%; 2013 IMS Health data [37]), SPCs, and Finnish clinical practice.
Cost-effectiveness analysis was performed for an “average patient” from the first bDMARD for moderate-to-severe RA over a period of 5 years. For the current treatment mix, the model simulated patients starting with each of the different bDMARDs, and then averaged these results on the basis of drug market share.
On the basis of reimbursement statistics from the Finnish Social Insurance Institution and sales data from IMS Health, we estimated that 799 RA patients received new first-line bDMARDs in 2015 (biologic-naïve patients, the initial target population for budget impact analysis), a number that is estimated to increase by 3% per annum until 2019 on the basis of historical trends. Patients were assumed to use the first-line bDMARD in combination with methotrexate (90% of bDMARD-treated Finnish patients with RA had received cDMARD treatment at bDMARD treatment initiation) [12].
In the compared settings, patients continued with bDMARD and methotrexate treatment beyond week 24 if the minimum response criterion of ACR20 was met and no adverse event precluding treatment continuation occurred (incidence of 1.9% during 24 weeks [38]). The maximum duration of first-line bDMARD treatment was set to 144 weeks. Patients discontinuing first-line bDMARD treatment were assumed to switch to rituximab, which has been shown to be a cost-effective subsequent treatment in Finnish settings [39, 40]. Patients exited the model through death (constant mortality rate adjusted for disease severity based on HAQ-DI score) or once they reached the model maximum of 260 weeks.
Clinical Inputs
Efficacy data for bDMARDs were estimated by a meta-analysis, applying a random effects model of efficacy responses from clinical trials, accounting for the correlations between outcomes, and separating outcomes reported at week 12 or 24 (see Appendix A in the electronic supplementary material). In the absence of long-term randomized data on treatment effects, and because of the intention-to-treat setting of clinical trials, the first-line treatment response (without adverse events) was assumed to persist until death or the modeled maximum timeframe of 144 weeks with the first-line treatment [12, 41].
ACR20 and ACR50 treatment outcomes for second-line rituximab treatment (infliximab treatment followed by rituximab treatment in a sensitivity analysis scenario) after initial bDMARD treatment were modeled on patient response and persistence data from the South Swedish Arthritis Treatment Group Register and the Spanish BIOBADASER database [41–43].
Within the analysis timeframe, patients had a constant mortality rate (annual rate of 3.05 per 1000 patient-years at age 52 years for 2013 in Finland [44, 45]). This was adjusted upward on the basis of the HAQ-DI status of the patient to compensate for the elevated risk of death associated with RA, given as an odds ratio of 2.93 (95% confidence interval 2.43–3.54) per unit increase in HAQ-DI score (based on this being the estimate with the best Bayesian information criterion and Z score among RA factors [46]). The impact of excluding elevated mortality due to RA was tested in sensitivity analyses.
At the patient level, the HAQ-DI score takes values in multiples of 0.125. However, the representative cohort’s initial mean HAQ-DI score was assumed to be 1.2 (standard deviation 0.7), in line with the values used in an earlier Finnish RA assessment of first-line bDMARDs (mean HAQ-DI score 1.2 [40]) among Finnish RA register bDMARD users (mean HAQ-DI score 1.1 [12]) and from mortality information (mean HAQ-DI score 1.2, standard deviation 0.76) [46]. HAQ-DI score was predicted to change in relation to ACR response level, as reported in an earlier Finnish HE analysis [40]. HAQ-DI score was not assumed to increase as a result of RA. For cost–utility outcomes, QoL was modeled with use of the published linear relationship between HAQ-DI and the Health Utility Index [47]. The impact of QoL values was tested in a sensitivity analysis using HAQ-DI bands.
Economic Inputs
A societal 5-year perspective was used in the analyses, including direct medical and traveling costs, and HCA-based productivity losses. Payer perspective results were also calculated (i.e., excluding the productivity losses). Input of drug administration was based on SPC guidelines. The cheapest reimbursed retail costs for drugs were sourced from the Finnish Medicines Tariff, June 2015 (including biosimilar infliximab, and subcutaneously administered abatacept and tocilizumab; Table 1).
The incidence of hospitalization was modeled according to HAQ-DI scores [48], and Finnish productivity losses were included on the basis of the reported association between ACR responses and work days lost [18]. The HCA was used to analyze the potential productivity losses, since ACR responses and HAQ-DI scores are important for the analysis of productivity losses in RA, and HCA-based productivity losses are associated with both HAQ-DI score [49] and ACR response [18].
Initiation of bDMARD treatment consisted of a nurse visit (for subcutaneously administered treatments only), an antibody test, an QuantiFERON test, and chest X-ray. Resource use for the 12-week treatment cycles included 0.5 GP visits, 0.5 outpatient visits, 1.5 laboratory visits and phone consultations [1.5 liver value tests (alanine aminotransferase), 1.5 blood counts, and 0.5 creatine tests], and related traveling. Intravenous administration costs were based on a Finnish study [50]. Unit costs are listed in Table 1. The potential extra specialist visit needed to assess the RSS response criterion at week 12 was also included.
Sensitivity Analyses
The sensitivity of the modeling assumptions was assessed in the following settings: no RSS for CZP, drug-to-drug comparisons for commonly used bDMARDs (100% adalimumab, etanercept, golimumab, or biosimilar infliximab treatment mix assumed); three bDMARDs modeled (infliximab included in the treatment sequence between the current mix or CZP and rituximab); inclusion of ACR70 response for the subsequent treatment; first-line treatment duration of 72 weeks; first-line treatment duration of 240 weeks; additional mortality due to RA ignored; QoL based on HAQ-DI bands [51]; no discounting; ACR response assessment at week 12, and ACR response assessment at week 24.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Clinical Outcomes
The clinical evidence network for meta-analysis comprised 30 studies (Fig. 2). The included trials, log odds ratios, their standard errors and correlations, and probabilities of superior ACR response are reported in Appendix A in the electronic supplementary material.
The CZP RSS (based on the assessment of response at week 12) was estimated to reduce the time in which no ACR response was seen by 5%-units, and increase the time with ACR20, ACR50, and ACR70 responses by 5%-, 6%-, and 1%-unit, respectively, when compared with the current treatment mix (response assessments at week 24).
Cost-effectiveness
In the cost-effectiveness analysis, CZP treatment was estimated to dominate the current treatment mix, as CZP increased QALYs by 0.03, reduced absenteeism from work by 24 days, and reduced costs by €7866 per patient over the 5 years when compared with the current treatment mix (Table 2). This remained consistent without the RSS (estimated €6381 savings per patient). Modeling results by timing of response assessments are reported in Appendix B in the electronic supplementary material.
The probability of the CZP RSS providing the highest net monetary benefit was estimated to be 97% at a willingness-to-pay threshold of €30,000 per QALY gained. The CZP RSS constituted the cost-effectiveness acceptability frontier (Fig. 4). Without the RSS, CZP treatment was estimated to have a high (91%) probability of being cost-effective at the same willingness-to-pay threshold. The per-patient impact of the RSS on the cost of CZP treatment was estimated to lead to savings of €1472, and increase the probability of cost-effectiveness by approximately 6%.
Budget Impact
At the population level, the budget impact analysis of CZP with the RSS was estimated to reduce costs by 1.7% in the first year and by 5.6% in 2019, compared with the current treatment mix. The reduction in productivity losses had the greatest contribution to these cost savings, with an estimated decrease in absent days ranging from approximately 3000 in the first year to 25,000 in 2019 (Table 3). Appendix C in the electronic supplementary material reports detailed results for the estimated budget impact analysis scenarios assuming different response assessment times (week 12 or 24), which demonstrated that response assessment at week 12 had the lowest budget impact for CZP. For the current treatment mix, response assessment at week 24 had the lowest budget impact.
The estimated acquisition cost of first-line bDMARDs increased by €3.2 million from 2015 to 2019 with the CZP RSS, mainly due to an increase in the number of patients. However, this cost increase was offset by €6.1 million of savings in other costs (e.g., productivity losses) in 2019. Of these estimated savings, the RSS refund represented only €1.5 million, with the largest potential for savings coming from reductions in productivity losses. The proportional estimated savings per patient (from 1.7% in 2015 to 5.7% in 2019) were in line with the findings over the total population. Without the RSS, the use of CZP in place of the current treatment mix was estimated to reduce the treatment costs by 0.6% in 2015 and by 4.6% in 2019 (the total budget would be reduced by €0.8 million in 2015 and by €6.6 million in 2019).
Sensitivity Analyses
The results of the sensitivity analyses (see Appendix D in the electronic supplementary material) are shown in Figs. 3 and 4. In the pairwise cost–utility comparison, CZP dominated other bDMARDs commonly used as single treatments (i.e., adalimumab, etanercept, golimumab, and biosimilar infliximab; Fig. 3) and the current treatment mix (Fig. 4) on the basis of outcomes of the CZP RSS. In addition, CZP was estimated to result in a lower budget and reduced loss of productivity when compared with commonly used bDMARDs.
Cost-effectiveness (a) and budget impact results (b) for key sensitivity analysis scenarios. Results based on week 12 or 24 clinical response assessment for all treatments are reported in Appendices B and C in the electronic supplementary material. CZP certolizumab pegol, HAQ Health Assessment Questionnaire, QALY quality-adjusted life year, QoL quality of life, RSS risk-sharing scheme
In drug-to-drug cost–utility modeling, etanercept was estimated to be the best option after CZP in terms of health outcomes, and biosimilar infliximab was second to CZP in terms of affordability. A longer duration of first-line bDMARD treatment (if feasible, given the need for treatment efficacy) or the addition of another first-line bDMARD (here biosimilar infliximab) resulted in improved average outcomes and increased the relative difference between CZP treatment and the current treatment mix (see Appendix D in the electronic supplementary material).
Discussion
In comparison with the current treatment mix of first-line reimbursed bDMARDs for RA, early risk sharing of CZP for biologic-naïve patients was estimated to lead to cost savings per patient, and to produce 1.7–5.6% of savings in the total annual health care budget for moderate-to-severe RA patients treated with bDMARDs in Finland from 2015 to 2019. Furthermore, CZP treatment with or without risk sharing was estimated to increase the total number of working days by 2971 in 2015 to 25,177 in 2019 annually in Finland, while increasing the duration of ACR response and QALYs gained.
CZP, both with and without the RSS, was estimated to have a high probability of cost-effectiveness (97% and 91%, respectively, at a willingness-to-pay threshold of €30,000 per QALY gained); these probabilities were robust over a range of international willingness-to-pay thresholds. This finding has practical implications, since payers’ willingness to pay per QALY gained has not been publicly announced in Finland [25]. When budget is fixed to the current mix (impact investment assessment [15]) and assuming that demand exceeds supply, the surplus provided by the CZP RSS could result in the treatment of approximately 6% more RA patients and 0.03 more QALYs gained per biologic-using, Finnish RA patient compared with the current mix.
The base case results of this modeling study were based on the most conservative response assessments for CZP (week 24 response assessment for the current treatment mix, week 12 response assessment for the CZP RSS). Sensitivity analyses showed the greatest estimated gains from CZP treatment with or without the RSS were obtained from a setting in which continuation of all options was dependent on week 12 ACR response. When week 12 response was used for all treatments in the sensitivity analysis, the base case estimated incremental benefits produced by the CZP RSS were approximately doubled. This further emphasizes the benefits of early response monitoring and assessment of RA patients, in line with the recent EULAR recommendations [35] and CZP labeling guidelines. This analysis demonstrated that early use of a CZP RSS has the potential to improve outcomes (due to the rapid response). On the basis of Finnish national administrative data, the initiation of active drug treatment in the first 3 months following RA diagnosis was associated with significantly lower cumulative productivity costs [52], which further highlights the value of early treatment.
The estimated impact of the RSS on CZP’s cost-effectiveness probability was approximately 6%, meaning that only a small proportion of the RSS HE benefits of CZP were explained by the RSS alone. This conclusion is supported by the dominance of CZP (with or without an RSS) over other commonly used bDMARDs in the individual (i.e., pairwise) treatment analyses. The results were driven instead by drug-related costs, treatment efficacy, and reductions in productivity losses.
A new hybrid-type HE model was built and used to synthesize the available evidence. An important source of information for the model was the new meta-analysis (see Appendix A in the electronic supplementary material), which is in agreement with recent findings reported elsewhere. For example, the meta-analysis of efficacy that informed the EULAR guidelines reported a risk ratio of 8.52 (95% confidence interval 4.49–16.15) for the ACR70 response for CZP with methotrexate, versus methotrexate alone, in methotrexate-treated incomplete responders [53]. Moreover, a recent multiple treatment comparison regression analysis demonstrated that CZP had the highest ACR50 response ranking, with or without concomitant cDMARD treatment [54].
Modeled analyses typically include unavoidable simplifications, predominantly due to data scarcity. Firstly, treatment sequences were not explicitly modeled. Instead, real-world evidence was used for the post-first-line treatment responses and persistence. The inclusion of a third treatment in the sequence had only a minor effect on the results in a sensitivity analysis. Secondly, mortality evidence was not exact; thus, the minor increase in the number of patients treated with CZP may be unrealistic. This was tested in sensitivity analysis, in which mortality input had only a minor impact on the results.
Productivity losses in the analyses were based on the association reported between ACR response and days absent from work, and not on patient well-being. By use of this approach, there was no need to include the avoidable uncertainty associated with modeling productivity losses indirectly through HAQ-DI. The HCA was preferred over the friction cost approach (FCA) for several reasons. The FCA limits the number of disability days, and may also significantly underestimate productivity losses [49] from the societal perspective. Furthermore, the FCA includes many unrealistic or difficult-to-assess assumptions (e.g., friction time; the perfect obtainability of the workforce, i.e., no labor shortage; the assumed fact that disability compensations are income transfers and have no value for society; the fact that no productivity loss is observed when the friction period for a working age person is complemented; and the lack of difference between unemployed and employed patients in terms of the societal value created after the friction period).
Unfortunately, our study lacked data related to presenteeism, which has constituted a major proportion of productivity associated costs in recent analyses including RA [55]. However, the cost of sickness absence during the first 3 months can exceed the HCA estimate because of sick-leave payments, and friction-based productivity losses decrease over time irrespective of any increase in the number of absent days [49], posing significant credibility issues for the FCA. Other studies have also considered the HCA to be a good reflection of the true economic impact of absenteeism [49] or of presenteeism, absenteeism, retraining, and lost work at home [55]. Thus, the productivity costs modeled here are likely to be underestimates irrespective of the HCA.
However, the payer perspective analysis, even when excluding productivity losses, indicates cost savings, despite the modeled reduction in productivity losses constituting most of the modeled total savings. The HAQ-DI score was conservatively assumed not to increase over time; however, if disease-related increase of the HAQ-DI score over time was assumed, the incremental cost-effectiveness and budget saving due to the CZP RSS would increase. Lastly, this modeled comparison was optimized with 100% uptake of CZP treatment, if available as part of an RSS; that is, the ideal savings presented here may not be realized in full for other bDMARDs if they were included in an RSS.
Despite the limitations discussed, this modeling study demonstrates novel results for a variety of important stakeholders. From a societal perspective—alongside health care payers, clinical decision-making bodies, and patients—the increased use of CZP with or without the RSS is desirable because it elicits an estimated reduction in both expenditure and subsequent tax burden, while simultaneously providing health benefit, increased productivity, and tax-based income. On the basis of the Finnish health care, social welfare, and regional government reform package [56], in which a primary target is to produce both effective and cost-effective social and health care services (and produce effectiveness and cost-effectiveness data for quality control and benchmarking), an RSS may prove useful, as has been mentioned previously [14]. Recently, conditional reimbursement has been included in parliamentary discussion [16, 17]. Additionally, a performance-based RSS could form part of a strategy that enables more patients to access effective treatments within a given budget. The drug manufacturer would be expected to refund 5.7% of the estimated annual sales of CZP in 2019.
Generally, the optimal decision between an RSS and no RSS depends, for example, on the trade-off between the monitoring costs, the marginal production cost, and the utility derived from the treatment [57], as well as uncertainty related to treatment effectiveness and nondrug incremental costs [58]. If the treatment imposes a high cost on the health care system, relative to the related monitoring costs, the health authority may prefer an RSS agreement. Such a case was modeled here, based on the data available. However, more studies are needed to model the outcomes of different treatment sequences and the real-world HE outcomes of an RSS, where implemented. HE/outcomes research studies and RSSs in Finland are likely to get easier because of the interest of current care working groups in HE issues [59], and the planned launch of Isaacus (in early 2018), a Finnish data operator that is expected to provide nationally representative well-being data from different information sources and registers on a one-stop-shop basis [60].
Conclusions
The hypothetical RSS modeled in this study could potentially improve the ACR response, QALY, and economic outcomes associated with moderate-to-severe RA in Finland. The study estimated that, with or without an RSS, CZP is a cost-effective and affordable treatment for patients with moderate-to-severe RA in Finland.
References
Barros PP. Pharmaceutical policies in European countries. Adv Health Econ Health Serv Res. 2010;22:3–27.
Carone G Schwierz C, Xavier A. Cost-containment policies in public pharmaceutical spending in the EU. Brussels: European Commission; 2012.
Cook JP, Vernon JA, Manning R. Pharmaceutical risk-sharing agreements. Pharmacoeconomics. 2008;26(7):551–6.
Adamski J, Godman B, Ofierska-Sujkowska G, et al. Risk sharing arrangements for pharmaceuticals: potential considerations and recommendations for European payers. BMC Health Serv Res. 2010;10(1):153.
Söderlin MK, Kautiainen H, Skogh T, Leirisalo-Repo M. Quality of life and economic burden of illness in very early arthritis. A population based study in southern Sweden. J Rheumatol. 2004;31(9):1717–22.
Saarni SI, Härkänen T, Sintonen H, et al. The Impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res. 2006;15(8):1403–14.
Sokka T, Kautiainen H, Pincus T, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. 2010;12(2):R42.
Gron KL, Ornbjerg LM, Hetland ML, et al. The association of fatigue, comorbidity burden, disease activity, disability and gross domestic product in patients with rheumatoid arthritis. Results from 34 countries participating in the Quest-RA program. Clin Exp Rheumatol. 2014;32(6):869–77.
Uhlig T, Moe RH, Kvien TK. The burden of disease in rheumatoid arthritis. Pharmacoeconomics. 2014;32(9):841–51.
Hakala M. Nivelreuman puhkeaminen ja yleisyys. In: Martio J, Karjalainen A, Kauppi M, Kukkurainen ML, Kyngäs H, editors. Rheumatoid disorders. Helsinki: Kustannus Oy Duodecim; 2007. p. 321–40.
Bossaller L, Rothe A. Monoclonal antibody treatments for rheumatoid arthritis. Expert Opin Biol Ther. 2013;13(9):1257–72.
Virkki L, Aaltonen K, Nordström D. Biologiset reumalääkkeet-käytännön kokemukset rekisteritulosten valossa. Duodecim. 2010;126(12):1487–95.
Nyblin K. Mihin suuntaan lääkekorvausjärjestelmä kehittyy? Helsinki: Defensor Legis; 2009; p. 6.
Soini E. Asiantuntijaratkaisuja vaikuttavuustutkimukseen. Lakityöryhmän WorkShop, SoTe-tiedon toissijainen käyttö: Tiedolla johtaminen, ohjaus ja valvonta. Helsinki: Ministry of Social Affairs and Health; 2016.
Soini E, Hautala A, Poikonen E, Becker U, Kyttälä M, Martikainen J. Cost-effectiveness of first-line chronic lymphocytic leukemia treatments when full-dose fludarabine is unsuitable. Clin Ther. 2016;38(4):889–904.
Eduskunta. Hallituksen esitys HE 184/2016 vp. 2016.
Pelkonen L. Ehdollinen korvattavuus. Hilan asiointipalvelu ja muutokset lääkekorvausasioissa. Helsinki: Pharmaceuticals Pricing Board; 2016.
Puolakka K, Kautiainen H, Pekurinen M, et al. Monetary value of lost productivity over a five year follow up in early rheumatoid arthritis estimated on the basis of official register data on patients’ sickness absence and gross income: experience from the FIN-RACo trial. Ann Rheum Dis. 2006;65(7):899–904.
Martikainen J, Hallinen T, Soini E. Economic evaluation of medicines. Pharmaceutical economics and management: theory, research and practice. Dosis. 2006;22:289–300.
Peura P, Martikainen J, Soini E. Cost-effectiveness of statins in the prevention of coronary heart disease events in middle-aged Finnish men. Curr Med Res Opin. 2008;24:1823–32.
Soini E, Davies G, Martikainen J. Population-based health-economic evaluation of the secondary prevention of coronary heart disease in Finland. Curr Med Res Opin. 2010;26:25–36.
Soini E, Martikainen J, Nousiainen T. Treatment of follicular non-Hodgkin’s lymphoma with or without rituximab: cost-effectiveness and value of information based on a 5-year follow-up. Ann Oncol. 2011;22:1189–97.
Soini E, Kukkonen J, Myllykangas M, Ryynänen O. Contingent valuation of eight new treatments: what is the clinician’s and politician’s willingness to pay? Open Complement Med J. 2012;4:1–11.
Soini E. Reuman hoidon kustannusvaikuttavuus Suomessa. Best Pract Reumasairaudet. 2013;1:7–9.
Soini EJ, Hallinen T, Sokka AL, Saarinen K. Cost-utility of first-line actinic keratosis treatments in Finland. Adv Ther. 2015;32(5):455–76.
National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
Collins M, Latimer N. NICE’s end of life decision making scheme: impact on population health. BMJ. 2013;346:f1363.
Fimea. Lääkkeiden HTA-neuvottelukunnan kokous 3/2014. Muistio. Helsinki: Lääkealan turvallisuus- ja kehittämiskeskus 20.10.2014.
Soini EJ, Kukkonen J, Myllykangas M, Ryynänen O-P. Contingent valuation of eight new treatments: what is the clinician’s and politician’s willingness to pay. Open Complement Med J. 2012;4:1–11.
Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
Lääkkeiden Hintalautakunta. Terveystaloudellisen selvityksen laatiminen lääkevalmisteen korvattavuus- ja tukkuhintahakemukseen. Helsinki: Pharmaceuticals Pricing Board; 2014.
Keystone EC, Curtis JR, Fleischmann RM, et al. Rapid improvement in the signs and symptoms of rheumatoid arthritis following certolizumab pegol treatment predicts better longterm outcomes: post hoc analysis of a randomized controlled trial. J Rheumatol. 2011;38(6):990–6.
Curtis JR, Luijtens K, Kavanaugh A. Predicting future response to certolizumab pegol in rheumatoid arthritis patients: features at 12 weeks associated with low disease activity at 1 year. Arthritis Care Res. 2012;64(5):658–67.
Takeuchi T, Yamamoto K, Yamanaka H, et al. Early response to certolizumab pegol predicts long-term outcomes in patients with active rheumatoid arthritis: results from the Japanese studies. Mod Rheumatol. 2015;25(1):11–20.
Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Reuma. Käypä hoito -suositus. Suomalaisen Lääkäriseuran Duodecimin ja Suomen Reumatologisen yhdistyksen asettama työryhmä. Helsinki: Suomalainen Lääkäriseura Duodecim; 2015.
IMS Dataview 7. 2006 IMS Health Incorporated.
Du Pan SM, Dehler S, Ciurea A, Ziswiler HR, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti–tumor necrosis factor agents in rheumatoid arthritis. Arthritis Care Res. 2009;61(5):560–8.
Hallinen TA, Soini EJ, Eklund K, Puolakka K. Cost–utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology. 2010;49(4):767–77.
Soini EJ, Hallinen TA, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ. 2012;15(2):340–51.
Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11(Suppl 1):S1.
Karlsson J, Kristensen LE, Kapetanovic M, Gülfe A, Saxne T, Geborek P. Treatment response to a second or third TNF-inhibitor in RA: results from the South Swedish Arthritis Treatment Group Register. Rheumatology. 2008;47(4):507–13.
Gomez-Reino JJ, Carmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8(1):1.
Suomen Virallinen Tilasto. Kuolleet. Helsinki: Tilastokeskus; 2014.
Suomen Virallinen Tilasto. Väestörakenne. Helsinki: Tilastokeskus; 2014.
Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(6):1530–42.
Bansback N, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis. 2005;64(7):995–1002.
Kobelt G, Eberhardt K, Joensson L, Jönsson B. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum. 1999;42(2):347–56.
Puolakka K, Kautiainen H, Möttönen T, Hannonen P, Korpela M, Hakala M, Luukkainen R, Vuori K, Blåfield H, Leirisalo-Repo M. Use of the Stanford Health Assessment Questionnaire in estimation of long-term productivity costs in patients with recent-onset rheumatoid arthritis. Scand J Rheumatol. 2009;38:96–103.
Soini EJ, Leussu M, Hallinen T. Administration costs of intravenous biologic drugs for rheumatoid arthritis. Springerplus. 2013;2(1):1.
Vera-Llonch M, Massarotti E, Wolfe F, et al. Cost–effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to methotrexate. Rheumatology. 2008;47(4):535–41.
Martikainen JA, Kautiainen H, Rantalaiho V, Puolakka KT. Longterm work productivity costs due to absenteeism and permanent work disability in patients with early rheumatoid arthritis: a nationwide register study of 7831 patients. J Rheumatol. 2016;43(12):2101–5.
Nam JL, Ramiro S, Gaujoux-Viala C, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2013 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2014;73(3):516–28.
Tvete IF, Natvig B, Gåsemyr J, Meland N, Røine M, Klemp M. Comparing effects of biologic agents in treating patients with rheumatoid arthritis: a multiple treatment comparison regression analysis. PLoS ONE. 2015;10(9):e0137258.
ESiOR Oy, Soini E.Tekemätöntä työtä, näkymättömiä kustannuksia—selvitys tulehduksellisia suolistosairauksia ja reumasairauksia sairastavien työ- ja toimintakyvystä sekä niiden menetyksestä aiheutuvista kustannuksista. Kuopio: ESiOR, 2017. Available from https://www.researchgate.net/publication/318420433_Tekematonta_tyota_nakymattomia_kustannuksia_-_Selvitys_tulehduksellisia_suolistosairauksia_ja_reumasairauksia_sairastavien_tyo-_ja_toimintakyvysta_seka_niiden_menetyksesta_aiheutuvista_kustannuksista. Accessed 14 Aug 2017.
Alueuudistus. Sote- ja aluehallintouudistus. Helsinki: Ministry of Social Affairs and Health, Ministry of Finance; 2016.
Antonanzas F, Juarez-Castello C, Rodriguez-Ibeas R. Should health authorities offer risk-sharing contracts to pharmaceutical firms? A theoretical approach. Health Econ Policy Law. 2011;6(3):391–403.
Zaric GS, Xie B. The impact of two pharmaceutical risk-sharing agreements on pricing, promotion, and net health benefits. Value Health. 2009;12(5):838–45.
Soini E. Biologisten lääkkeiden kustannusvaikuttavuus nivelpsoriaasin hoidossa. Helsinki: Suomalainen Lääkäriseura Duodecim, 2017. http://www.kaypahoito.fi/web/kh/suositukset/suositus?id=nix02465&suositusid=hoi500. Accessed 14 Aug 2017.
Soini E, Hallinen T, Kekoni A, Kotimaa J, Nykänen M, Tirkkonen J, Tervahauta M. Efficient secondary use of representative social and health care data in Finland: Isaacus data lake, analytics and knowledge management pre-production project. Value Health. 2017;20 (in press).
Kapiainen S, Väisänen A, Haula T. Terveyden-ja sosiaalihuollon yksikkökustannukset Suomessa vuonna. 2011. Tampere: Terveyden ja hyvinvoinnin laitos (THL); 2014.
Hujanen T, Kapiainen S, Tuominen U, Pekurinen M. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006. Helsinki: Stakes; 2008.
Suomen Virallinen Tilasto. Kuluttajahintaindeksi. Helsinki: Tilastokeskus; 2014.
Suomen Virallinen Tilasto. Julkisten menojen hintaindeksi. Helsinki: Tilastokeskus; 2014.
Acknowledgements
Sponsorship for this study and article processing charges were funded by UCB Pharma Oy Finland, Espoo, Finland.
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
We are grateful to Taru Hallinen from ESiOR Oy, and Lisa Wulund, Simon Page, Jade Ogle, and Sam Fraser from Costello Medical Consulting for support with medical writing. Selected sections of the results were presented at the 15th International Society for Pharmacoeconomics and Outcomes Research Annual European Congress (Asseburg C, Soini E, Taiha M. Value Health. 2012;15:A454), and the European League Against Rheumatism 2013 Congress [Asseburg C, Soini E, Puolakka K, Purcaru O, Taiha M, Luosujärvi R. Ann Rheum Dis. 2013;72(Suppl 3):434].
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Erkki Soini is employed by and holds shares in ESiOR Oy, which carries out health economic consultancy, analysis, and studies for other organizations. Christian Asseburg was an employee and shareholder in ESiOR Oy at the time of analysis. Maarit Taiha is employed by UCB Pharma Oy Finland. Oana Purcaru is employed by UCB Pharma SA Belgium. Riitta Luosujärvi and Kari Puolakka declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Contributions
Study management Erkki Soini and Maarit Taiha; concept and study/analysis plan Erkki Soini, Maarit Taiha, Christian Asseburg, Riitta Luosujärvi, Kari Puolakka, and Oana Purcaru; data assembly Christian Asseburg, Erkki Soini, Maarit Taiha, Riitta Luosujärvi, Kari Puolakka, and Oana Purcaru; analysis Christian Asseburg and Erkki Soini; manuscript drafting Erkki Soini; critical manuscript revision Erkki Soini, Christian Asseburg, Riitta Luosujärvi, Kari Puolakka, Maarit Taiha, and Oana Purcaru.
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/0A0CF060409F19DA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Soini, E., Asseburg, C., Taiha, M. et al. Modeled Health Economic Impact of a Hypothetical Certolizumab Pegol Risk-Sharing Scheme for Patients with Moderate-to-Severe Rheumatoid Arthritis in Finland. Adv Ther 34, 2316–2332 (2017). https://doi.org/10.1007/s12325-017-0614-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0614-8