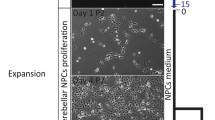
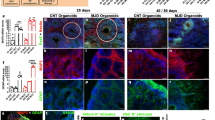
Abstract
Recent advances in the techniques that differentiate induced pluripotent stem cells (iPSCs) into specific types of cells enabled us to establish in vitro cell-based models as a platform for drug discovery. iPSC-derived disease models are advantageous to generation of a large number of cells required for high-throughput screening. Furthermore, disease-relevant cells differentiated from patient-derived iPSCs are expected to recapitulate the disorder-specific pathogenesis and physiology in vitro. Such disease-relevant cells will be useful for developing effective therapies. We demonstrated that cerebellar tissues are generated from human PSCs (hPSCs) in 3D culture systems that recapitulate the in vivo microenvironments associated with the isthmic organizer. Recently, we have succeeded in generation of spinocerebellar ataxia (SCA) patient-derived Purkinje cells by combining the iPSC technology and the self-organizing stem cell 3D culture technology. We demonstrated that SCA6-derived Purkinje cells exhibit vulnerability to triiodothyronine depletion, which is suppressed by treatment with thyrotropin-releasing hormone and Riluzole. We further discuss applications of patient-specific iPSCs to intractable cerebellar disease.

Similar content being viewed by others
References
Muguruma K, Sasai Y. In vitro recapitulation of neural development using embryonic stem cells: from neurogenesis to histogenesis. Develop Growth Differ. 2012;54(3):349–57. https://doi.org/10.1111/j.1440-169X.2012.01329.x.
Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520–30. https://doi.org/10.1016/j.stem.2013.04.009.
Muguruma K, Nishiyama A, Ono Y, Miyawaki H, Mizuhara E, Hori S, et al. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nature Neurosci. 2010;13(10):1171–80. https://doi.org/10.1038/nn.2638.
Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10(4):537–50. https://doi.org/10.1016/j.celrep.2014.12.051.
Joyner AL, Liu A, Millet S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol. 2000;12(6):736–41. https://doi.org/10.1016/S0955-0674(00)00161-7.
Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nature. Rev Neurosci. 2001;2(2):99–108. https://doi.org/10.1038/35053516.
Martinez S, Andreu A, Mecklenburg N, Echevarria D. Cellular and molecular basis of cerebellar development. Front Neuroanat. 2013;7:1–12. https://doi.org/10.3389/fnana.2013.00018.
Marzban H, Del Bigio MR, Alizadeh J, Ghavami S, Zachariah RM, Rastegar M. Cellular commitment in the developing cerebellum. Front Cell Neurosci. 2015;18:450. https://doi.org/10.3389/fncel.2014.00450.
Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14(1):91–100. https://doi.org/10.1177/1073858407304629.
Leto K, Arancillo M, Becker EBE, Buffo A, Chiang C, Ding B, et al. Consensus paper: cerebellar development. Cerebellum. 2016;15(6):789–828. https://doi.org/10.1007/s12311-015-0724-2.
Watson LM, Wong MMK, Becker EBE. Induced pluripotent stem cell technology for modeling and therapy of cerebellar ataxia. Open Biol. 2015;5(7):150056. https://doi.org/10.1098/rsob.150056.
Rüb U, Schöls L, Paulson H, Auburger G, Kermer P, Jen JC, et al. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1,2,3,6 and 7. Prog Neurobiol. 2013;104:38–66. https://doi.org/10.1016/j.pneurobio.2013.01.001.
Ishida Y, Kawakami H, Kitajima H, Nishiyama A, Sasai Y, Inoue H, et al. Vulnerability of Purkinje cells generated from spinocerebellar ataxia type 6 patient-derived iPSCs. Cell Rep. 2016;17(6):1482–90. https://doi.org/10.1016/j.celrep.2016.10.026.
Du X, Wang J, Zhu H, Rinaldo L, Lamar KM, Palmenberg AC, et al. Second cistron in CACNA1A gene incodes a transcription factor mediating cerebellar development and SCA6. Cell. 2013;154(1):118–33. https://doi.org/10.1016/j.cell.2013.05.059.
Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM. C-termini of P/Q-type Ca2+ channel α1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet. 2006;15(10):1587–99. https://doi.org/10.1093/hmg/ddl080.
Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nature Rev Drug Discov. 2017;16:115–30. https://doi.org/10.1038/nrd.2016.245.
Ardhanareeswaran K, Mariani J, Coppola G, Abyzov A, Vaccarino FM. Human induced pluripotent stem cells for modeling neurodevelopmental disorders. Nature Rev Neurol. 2017;13(5):265–78. https://doi.org/10.1038/nrneurol.2017.45.
Ku S, Soragni E, Campau E, Thomas EA, Altun G, Laurent LC, et al. Friedreich’s ataxia induced pluripotent stem cells model intergenerational GAA∙TTC triplet repeat instability. Cell Stem Cell. 2010;7(5):631–7. https://doi.org/10.1016/j.stem.2010.09.014.
Koch P, Breuer P, Peitz M, Jungverdorben J, Kesavan J, Poppe D, et al. Excitation-induced ataxin-3 aggregation in neurons from patients with Machado-Joseph disease. Nature. 2011;480(7378):543–6. https://doi.org/10.1038/nature10671.
Hick A, Wattenhofer-Donze M, Chintawar S, Tropel P, Simard JP, Vaucamps N, et al. Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich’s ataxia. Dis Model Mech. 2013;6(3):608–21. https://doi.org/10.1242/dmm.010900.
Bird MJ, Needham K, Frazier AE, van Rooijen J, Leung J, Hough S, et al. Functional characterization of Friedreich ataxia iPS derived neuronal progenitors and their integration on the adult brain. PLoS One. 2014;9(7):e101718. https://doi.org/10.1371/journal.pone.0101718.
Bavassano C, Eigentler A, Stanika R, Obermair GJ, Boesch S, Dechant G, et al. Bicistronic CACNA1A gene expression in neurons derived from spinocerebellar ataxia type 6 patient-induced pluripotent stem cells. Stem Cells Dev. 2017;26(22):1612–25. https://doi.org/10.1089/scd.2017.0085.
Klockgether T. Update on degenerative ataxias. Curr Opin Neurol. 2011;24(4):339–245. https://doi.org/10.1097/WCO.0b013e32834875ba.
Manto M, Marmolino D. Cerebellar ataxias. Curr Opin Neurol. 2009;22(4):419–29. https://doi.org/10.1097/WCO.0b013e32832b9897.
Morino H, Matsuda Y, Muguruma K, Miyamoto R, Ohsawa R, Ohtake T, et al. A mutation in the low voltage-gated calcium channel CACNA1G alters the physiological properties of the channel, causing spinocerebellar ataxia. Molecular Brain. 2015;8(1):89. https://doi.org/10.1186/s13041-015-0180-4.
Acknowledgements
This work was supported by grant-in-aid from Ministry of Health, Labour and Welfare, grant-in-aid for Scientific Research (C) from Japan Society for the Promotion of Science (JSPS), and the Core Program for Disease Modeling using iPS Cells from JST and AMED.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The author declares that she has no competing interests.
Rights and permissions
About this article
Cite this article
Muguruma, K. Self-Organized Cerebellar Tissue from Human Pluripotent Stem Cells and Disease Modeling with Patient-Derived iPSCs. Cerebellum 17, 37–41 (2018). https://doi.org/10.1007/s12311-017-0905-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-017-0905-2