Abstract
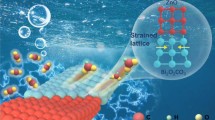
Surface strain engineering is considered as an effective strategy to promote the electrocatalytic properties of noble metal nanocrystals. Herein, we construct a dual-phase palladium-copper (DP-PdCu) bimetallic electrocatalyst with remarkable biaxial strain via a one-pot wet-chemical approach for formic acid oxidation. The biaxial strain originates from the lattice mismatch between the disordered face-centered cubic (FCC) phase and ordered body-centered cubic (BCC) phase in each of DP-PdCu nanoparticles. The proportion of FCC and BCC phases and size of PdCu nanoparticles are dependent on the addition amount of capping agent, cetyltrimethylammonium bromide (CTAB). Density functional theory calculations reveal the downshift of d-band center of Pd atoms due to the interfacial strain, which weakens the adsorption strength of undesired intermediates. These merit the DP-PdCu catalyst with superior mass activity of 0.55 A·mgPd-1 and specific activity of 1.91 mA·cmPd-2 toward formic acid oxidation, outperforming the single FCC/BCC PdCu and commercial Pd/C catalysts. This will provide new insights into the structure design of high-performance electrocatalysts via strain engineering.

Similar content being viewed by others
References
Chen, A. C.; Ostrom, C. Palladium-based nanomaterials: Synthesis and electrochemical applications. Chem. Rev. 2015, 115, 11999–12044.
Zhang, H.; Jin, M. S.; Xiong, Y. J.; Lim, B.; Xia, Y. N. Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Acc. Chem. Res. 2013, 46, 1783–1794.
Jin, M. S.; Zhang, H.; Xie, Z. X.; Xia, Y. N. Palladium nanocrystals enclosed by {100} and {111} facets in controlled proportions and their catalytic activities for formic acid oxidation. Energy Environ. Sci. 2012, 5, 6352–6357.
Wang, J. Y.; Zhang, H. X.; Jiang, K.; Cai, W. B. From HCOOH to CO at Pd electrodes: A surface-enhanced infrared spectroscopy study. J. Am. Chem. Soc. 2011, 133, 14876–14879.
Jiang, K.; Zhang, H. X.; Zou, S. Z.; Cai, W. B. Electrocatalysis of formic acid on palladium and platinum surfaces: From fundamental mechanisms to fuel cell applications. Phys. Chem. Chem. Phys. 2014, 16, 20360–20376.
Gilroy, K. D.; Ruditskiy, A.; Peng, H. C.; Qin, D.; Xia, Y. N. Bimetallic nanocrystals: Syntheses, properties, and applications. Chem. Rev. 2016, 116, 10414–10472.
Luo, M. C.; Zhao, Z. L.; Zhang, Y. L.; Sun, Y. J.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y. N. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85.
Jiang, K. Z.; Wang, P. T.; Guo, S. J.; Zhang, X.; Shen, X.; Lu, G.; Su, D.; Huang, X. Q. Ordered PdCu-based nanoparticles as bifunctional oxygen-reduction and ethanol-oxidation electrocatalysts. Angew. Chem., Int. Ed. 2016, 55, 9030–9035.
Geng, J. R.; Zhu, Z.; Bai, X. X.; Li, F. J.; Chen, J. Hot-injection synthesis of PtCu3 concave nanocubes with high-index facets for electrocatalytic oxidation of methanol and formic acid. ACS Appl. Energy Mater. 2020, 3, 1010–1016.
Ma, S. C.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R. T.; Gold, J. I.; Yamauchi, M.; Kenis, P. J. A. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu-Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 2017, 139, 47–50.
Zhu, Z.; Shi, X. M.; Fan, G. L.; Li, F. J.; Chen, J. Photo-energy conversion and storage in an aprotic Li-O2 battery. Angew. Chem., Int. Ed. 2019, 58, 19021–19026.
Hu, G. F.; Shang, L.; Sheng, T.; Chen, Y. G.; Wang, L. Y. PtCo@NCs with short heteroatom active site distance for enhanced catalytic properties. Adv. Funct. Mater. 2020, 30, 2002281.
Wang, C. Y.; Chen, D. P.; Sang, X. H.; Unocic, R. R.; Skrabalak, S. E. Size-dependent disorder-order transformation in the synthesis of monodisperse intermetallic PdCu nanocatalysts. ACS Nano 2016, 10, 6345–6353.
Zhou, M.; Li, C.; Fang, J. Y. Noble-metal based random alloy and intermetallic nanocrystals: Syntheses and applications. Chem. Rev. 2021, 121, 736–795.
Tong, W.; Huang, B. L.; Wang, P. T.; Li, L. G.; Shao, Q.; Huang, X. Q. Crystal-phase-engineered PdCu electrocatalyst for enhanced ammonia synthesis. Angew. Chem., Int. Ed. 2020, 59, 2649–2653.
Li, J. R.; Sun, S. H. Intermetallic nanoparticles: Synthetic control and their enhanced electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025.
Wang, D. L.; Xin, H. L.; Hovden, R.; Wang, H. S.; Yu, Y. C.; Muller, D. A.; DiSalvo, F. J.; Abruña, H. D. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87.
Kim, D.; Xie, C. L.; Becknell, N.; Yu, Y.; Karamad, M.; Chan, K. R.; Crumlin, E. J.; Nørskov, J. K.; Yang, P. D. Electrochemical activation of CO2 through atomic ordering transformations of AuCu nanoparticles. J. Am. Chem. Soc. 2017, 139, 8329–8336.
Zhao, X. R.; Cheng, H.; Song, L.; Han, L. L.; Zhang, R.; Kwon, G.; Ma, L.; Ehrlich, S. N.; Frenkel, A. I.; Yang, J. et al. Rhombohedral ordered intermetallic nanocatalyst boosts the oxygen reduction reaction. ACS Catal. 2021, 11, 184–192.
Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C. F.; Liu, Z. C.; Kaya, S.; Nordlund, D.; Ogasawara, H. et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nat. Chem. 2010, 2, 454–460.
Wang, T. Y.; Liang, J. S.; Zhao, Z. L.; Li, S. Z.; Lu, G.; Xia, Z. C.; Wang, C.; Luo J. H.; Han, J. T.; Ma, C. et al. Sub-6 nm fully ordered L10-Pt-Ni-Co nanoparticles enhance oxygen reduction via Co doping induced ferromagnetism enhancement and optimized surface strain. Adv. Energy Mater. 2019, 9, 1803771.
Liang, J. S.; Ma, F.; Hwang, S.; Wang, X. X.; Sokolowski, J.; Li, Q.; Wu, G.; Su, D. Atomic arrangement engineering of metallic nanocrystals for energy-conversion electrocatalysis. Joule 2019, 3, 956–991.
Huang, H. W.; Jia, H. H; Liu, Z.; Gao, P. F.; Zhao, J. T.; Luo, Z. L.; Yang, J. L.; Zeng, J. Understanding of strain effects in the electrochemical reduction of CO2: Using Pd nanostructures as an ideal platform. Angew. Chem., Int. Ed. 2017, 56, 3594–3598.
Wang, L.; Zeng, Z. H.; Gao, W. P.; Maxson, T.; Raciti, D.; Giroux, M.; Pan, X. Q.; Wang, C.; Greeley, J. Tunable intrinsic strain in twodimensional transition metal electrocatalysts. Science 2019, 363, 870–874.
Lu, Q. P.; Wang, A. L.; Gong, Y.; Hao, W.; Cheng, H. F.; Chen, J. Z.; Li, B.; Yang, N. L.; Niu, W. X.; Wang, J. et al. Crystal phase-based epitaxial growth of hybrid noble metal nanostructures on 4H/fcc Au nanowires. Nat. Chem. 2018, 10, 456–461.
Wang, H. T.; Xu, S. C.; Tsai, C.; Li, Y. Z.; Liu, C.; Zhao, J.; Liu, Y. Y.; Yuan, H. Y.; Abild-Pedersen, F.; Prinz, F. B. et al. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science 2016, 354, 1031–1036.
Li, M. F.; Zhao, Z. P.; Cheng, T.; Fortunelli, A.; Chen, C. Y.; Yu, R.; Zhang, Q. H.; Gu, L.; Merinov, B. V.; Lin, Z. Y. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419.
Li, W. D.; Zhao, Y. X.; Liu, Y.; Sun, M. Z.; Waterhouse, G. I. N.; Huang, B. L.; Zhang, K.; Zhang, T. R.; Lu, S. Y. Exploiting Ru-induced lattice strain in CoRu nanoalloys for robust bifunctional hydrogen production. Angew. Chem., Int. Ed. 2021, 60, 3290–3298.
Bu, L. Z.; Zhang, N.; Guo, S. J.; Zhang, X.; Li, J.; Yao, J. L.; Wu, T.; Lu, G.; Ma, J. Y.; Su, D. et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414.
Feng, Q. C.; Zhao, S.; He, D. S.; Tian, S. B.; Gu, L.; Wen, X. D.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Strain engineering to enhance the electrooxidation performance of atomic-layer Pt on intermetallic Pt3Ga. J. Am. Chem. Soc. 2018, 140, 2773–2776.
Li, J. R.; Sharma, S.; Wei, K. C.; Chen, Z. T.; Morris, D.; Lin, H. H.; Zeng, C.; Chi, M. F.; Yin, Z. Y.; Muzzio, M. et al. Anisotropic strain tuning of L10 ternary nanoparticles for oxygen reduction. J. Am. Chem. Soc. 2020, 142, 19209–19216.
Tan, X. Y.; Geng, S. Z.; Ji, Y. J.; Shao, Q.; Zhu, T.; Wang, P. T.; Li, Y. Y.; Huang, X. Q. Closest packing polymorphism interfaced metastable transition metal for efficient hydrogen evolution. Adv. Mater. 2020, 32, 2002857.
Yan, Y. C.; Du, J. S.; Gilroy, K. D.; Yang, D. R.; Xia, Y. N.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29, 1605997.
Gamler, J. T. L.; Ashberry, H. M.; Skrabalak, S. E.; Koczkur, K. M. Random alloyed versus intermetallic nanoparticles: A comparison of electrocatalytic performance. Adv. Mater. 2018, 30, 1801563.
Huang, F.; Banfield, J. F. Size-dependent phase transformation kinetics in nanocrystalline ZnS. J. Am. Chem. Soc. 2005, 127, 4523–4529.
Nørskov, J. K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943.
Nørskov, J. K.; Bligaard, T.; Rossmeisl, J.; Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46.
Wakisaka, M.; Mitsui, S.; Hirose, Y.; Kawashima, K.; Uchida, H.; Watanabe, M. Electronic structures of Pt-Co and Pt-Ru alloys for CO-tolerant anode catalysts in polymer electrolyte fuel cells studied by EC-XPS. J. Phys. Chem. B 2006, 110, 23489–23496.
Duchesne, P. N.; Li, Z. Y.; Deming, C. P.; Fung, V.; Zhao, X. J.; Yuan, J.; Regier, T.; Aldalbahi, A.; Almarhoon, Z.; Chen, S. W. et al. Golden single-atomic-site platinum electrocatalysts. Nat. Mater. 2018, 17, 1033–1039.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2016YFB0101201), the National Natural Science Foundation of China (Nos. 21822506 and 51761165025) and the 111 project of B12015.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Geng, J., Zhu, Z., Ni, Y. et al. Biaxial strained dual-phase palladium-copper bimetal boosts formic acid electrooxidation. Nano Res. 15, 280–284 (2022). https://doi.org/10.1007/s12274-021-3471-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3471-3