Abstract
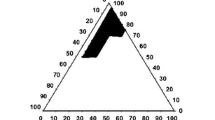
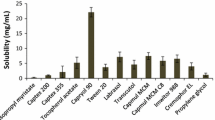
The purpose of this study was to develop a novel tacrolimus-loaded solid self-emulsifying drug delivery system (SEDDS) using Labrafac as an oil phase. The ternary phase diagram was plotted with Labrafac, Labrasol and Lauroglycol used as an oil, surfactant and co-surfactant, respectively. The liquid SEDDS formulated with Labrasol, Lauroglycol and Labrafac (70:15:15, volume ratio) furnished the smallest emulsion globule size. The solid SEDDS was obtained by spray-drying the liquid mixture containing the liquid SEDDS with 5 % tacrolimus and silicon dioxide. Furthermore, dissolution of tacrolimus from the solid SEDDS and pharmacokinetics in rats was studied compared to the commercial product. The solid SEDDS produced relatively larger emulsion globule size than that exhibited by the corresponding liquid SEDDS. However, this size variation was not significantly different. The solid SEDDS with approximately 280 nm emulsion droplet size improved the dissolution of the drug compared to drug power and the commercial product. It resulted in significantly higher plasma concentration, AUC and Cmax, and shorter Tmax values than did the commercial product (p < 0.05). The enormously enhanced oral bioavailability of tacrolimus in rats was attributed to relatively faster absorption due to accelerated dissolution of the drug from the solid SEDDS. Therefore, this novel solid SEDDS prepared with Labrafac as an oil phase is an excellent way to achieve better bioavailability of tacrolimus given via the oral route.





Similar content being viewed by others
References
Balakrishnan, P., B.J. Lee, D.H. Oh, J.O. Kim, M.J. Hong, J.P. Jee, J.A. Kim, B.K. Yoo, J.S. Woo, C.S. Yong, and H.G. Choi. 2009a. Enhanced oral bioavailability of dexibuprofen by a novel solid self-nanoemulsifying drug delivery system (SEDDS). European Journal of Pharmaceutics and Biopharmaceutics 72: 539–545.
Balakrishnan, P., B.J. Lee, D.H. Oh, J.O. Kim, Y.I. Lee, D.D. Kim, J.P. Jee, Y.B. Lee, J.S. Woo, C.S. Yong, and H.G. Choi. 2009b. Enhanced oral bioavailability of Coenzyme Q10 by self-emulsifying drug delivery systems. International Journal of Pharmaceutics 374: 66–72.
Borhade, V., H. Nair, and D. Hegde. 2008. Design and evaluation of self-microemulsifying drug delivery system (SMEDDS) of tacrolimus. AAPS Pharmaceutical Sciences and Technology 9: 13–21.
Chung, Y., and H. Cho. 2004. Preparation of highly water soluble tacrolimus derivatives: poly (ethylene glycol) esters as potential prodrugs. Archives of Pharmacal Research 27: 878–883.
Gao, S., J. Sun, D. Fu, H. Zhao, M. Lan, and F. Gao. 2012. Preparation, characterization and pharmacokinetic studies of tacrolimus-dimethyl-β-cyclodextrin inclusion complex-loaded albumin nanoparticles. International Journal of Pharmaceutics 427: 410–416.
Han, J., A. Beeton, P. Long, I. Wong, and C. Tuleu. 2006. Physical and microbiological stability of an extemporaneous tacrolimus suspension for paediatric use. Journal of Clinical Pharmacy and Therapeutics 31: 167–172.
Joe, J.H., W.M. Lee, Y.J. Park, K.H. Joe, D.H. Oh, Y.G. Seo, J.S. Woo, C.S. Yong, and H.G. Choi. 2010. Effect of the solid-dispersion method on the solubility and crystalline property of tacrolimus. International Journal of Pharmaceutics 395: 161–166.
Kang, J.H., D.H. Oh, Y.K. Oh, C.S. Yong, and H.G. Choi. 2012. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). European Journal of Pharmaceutics and Biopharmaceutics 80(2): 289–297.
Nishikawa, T., H. Hasumi, S. Suzuki, H. Kubo, and H. Ohtani. 1993. Kinetic analysis of molecular interconversion of immunosuppressant FK 506 by High-Performance Liquid Chromatography. Pharmaceutical Research 10: 1785–1789.
Oh, D.H., P. Balakrishnan, Y.K. Oh, D.D. Kim, C.S. Yong, and H.G. Choi. 2011. Effect of process parameters on nanoemulsion droplet size and distribution in SPG membrane emulsification. International Journal of Pharmaceutics 14: 191–197.
Oh, D.H., J.H. Kang, D.W. Kim, B.J. Lee, J.O. Kim, C.S. Yong, and H.G. Choi. 2012. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. International Journal of Pharmaceutics 420(2): 412–418.
Park, S.I., C.R. Felipe, P.G. Pinheiro-Machado, R. Garcia, H. Tedesco-Silva Jr, and J.O. Medina-Pestana. 2007. Circadian and time-dependent variability in tacrolimus pharmacokinetics. Fundamental and Clinical Pharmacology 21: 191–197.
Park, Y.J., D.S. Ryu, D.X. Li, Q.Z. Quan, D.H. Oh, J.O. Kim, Y.G. Seo, Y.I. Lee, C.S. Yong, J.S. Woo, and H.G. Choi. 2009. Physicochemical characterization of tacrolimus-loaded solid dispersion with sodium carboxylmethyl cellulose and sodium lauryl sulfate. Archives of Pharmacal Research 32(6): 893–898.
Shin, S.B., H.Y. Cho, D.D. Kim, H.G. Choi, and Y.B. Lee. 2010. Preparation and evaluation of tacrolimus-loaded nanoparticles for lymphatic delivery. European Journal of Pharmaceutics and Biopharmaceutics 74: 164–171.
Sinswat, P., K.A. Overhoff, J.T. McConville, K.P. Johnston, and R.O. Williams 3rd. 2008. Nebulization of nanoparticulate amorphous or crystalline tacrolimus-single-dose pharmacokinetics study in mice. European Journal of Pharmaceutics and Biopharmaceutics 69(3): 1057–1066.
Society of Toxicology (SOT). 2008. Guiding principles in the use of animals in toxicology. www.toxicology.org/AI/FA/guidingprinciples.pdf. Accessed in December.
Spencer, C.M., K.L. Goa, and J.C. Gillis. 1997. Tacrolimus. An update of its pharmacology and clinical efficacy in the management of organ transplantation. Drugs 54: 925–975.
Tran, T.H., B.K. Poudel, N. Marasini, S.C. Chi, H.G. Choi, C.S. Yong, and J.O. Kim. 2013. Preparation and evaluation of raloxifene-loaded solid dispersion nanoparticle by spray-drying technique without an organic solvent. International Journal of Pharmaceutics 443: 50–57.
Wang, Y., J. Sun, T. Zhang, H. Liu, F. He, and Z. He. 2011. Enhanced oral bioavailability of tacrolimus in rats by self-microemulsifying drug delivery systems. Drug Development and Industrial Pharmacy 37(10): 1225–1230.
Watts, A.B., A.M. Cline, A.R. Saad, S.B. Johnson, J.I. Peters, and R.O. Williams 3rd. 2010. Characterization and pharmacokinetic analysis of tacrolimus dispersion for nebulization in a lung transplanted rodent model. International Journal of Pharmaceutics 384: 46–52.
Yan, Y.D., J.H. Sung, K.K. Kim, D.W. Kim, J.O. Kim, B.J. Lee, C.S. Yong, and H.G. Choi. 2012. Novel valsartan-loaded solid dispersion with enhanced bioavailability and no crystalline changes. International Journal of Pharmaceutics 422: 202–210.
Yeo, W.H., T. Ramasamy, D.W. Kim, H.J. Cho, Y.I. Kim, K.H. Cho, C.S. Yong, J.O. Kim, and H.G. Choi. 2013. Docetaxel-loaded thermosensitive liquid suppository: optimization of rheological properties. Archives of Pharmacal Research 36: 1480–1486.
Acknowledgments
This work was supported by the research fund of Hanyang University (HY-2013-N).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Youn Gee Seo, Dong-Wuk Kim have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Seo, Y.G., Kim, DW., Cho, K.H. et al. Preparation and pharmaceutical evaluation of new tacrolimus-loaded solid self-emulsifying drug delivery system. Arch. Pharm. Res. 38, 223–228 (2015). https://doi.org/10.1007/s12272-014-0459-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0459-5