Abstract
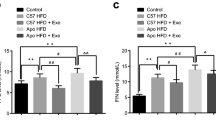
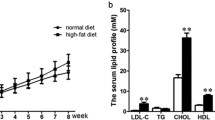
LncRNA microarray analysis was applied to investigate the exercise effect on the global differential expressions of lncRNA and mRNA in aorta endothelium in insulin resistance. Twenty-four male C57BL/6 J mice were randomly divided into three groups: control group (n = 8), high-fat diet group (n = 8), and high-fat diet plus exercise training group (n = 8). An lncRNA microarray analysis was applied to investigate the global differential expressions of lncRNA and mRNA in aorta endothelium among three groups and the results were further verified by qPCR. Hypergeometric distribution analysis was applied to reveal the possible signaling. Exercise might alleviate the vascular injury of IR via FR030200-Col3A1, FR402720-Rnd1, and FR030200/FR402720-Rnd3 signalings in cytoskeletal rearrangement pathway; E2F1-FR030200/FR402720-Nnat and FR030200/ FR402720-Fam46a signalings in anti-inflammation pathway. This study identified a panel of dysregulated lncRNAs and mRNAs that might serve as potential biomarkers relevant to the vascular injury in insulin resistance induced by high-fat diet.







Similar content being viewed by others
References
The Geriatrics Branch of Chinese Medical Association, Editorial Board of Chinese Journal of Internal Med, Editorial Board of Chinese Journal of Geriatrics. (2016). Aspirin use in patients with atherosclerotic cardiovascular disease: the 2016 Chinese expert consensus statement. Chinese Journal Geriatrics, 1, 106–118.
Volgman, A. S., Palaniappan, L. S., Aggarwal, N. T., et al. (2018). American Heart Association Council on Epidemiology and Prevention; Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation, 138(1), e1–e34.
Hanley, A. J., Williams, K., Stern, M. P., et al. (2002). Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care, 25(7), 1177–1184.
Rutter, M. K., Wilson, P. W., Sullivan, L. M., et al. (2008). Use of alternative thresholds defining insulin resistance to predict incident type 2 diabetes mellitus and cardiovascular disease. Circulation, 117(8), 1003–1009.
Janus, A., Szahidewiczkrupska, E., Mazur, G., et al. (2016). Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators of Inflammation, 2016(1), 1–10.
Patel, T. P., Rawal, K., Bagchi, A. K., et al. (2015). Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Failure Reviews, 21(1), 11–23.
Kraegen, E. W., Storlien, L. H., Jenkins, A. B., et al. (1989). Chronic exercise compensates for insulin resistance induced by a high-fat diet in rats. The American Journal of Physiology, 256(1), 242–249.
Rao, X., Zhong, J., Xu, X., et al. (2013). Exercise protects against diet-induced insulin resistance through downregulation of protein kinase cβ in mice. PLoS One, 8(12), e81364.
Park, D. R., Park, K. H., Kim, B. J., et al. (2015). Exercise ameliorates insulin resistance via Ca2+ signals distinct from those of insulin for GLUT4 translocation in skeletal muscles. Diabetes, 64(4), 1224.
Ying, C., Suixin, L., Kangling, X., et al. (2014). MicroRNA-492 reverses high glucose-induced insulin resistance in HUVsEC cells through targeting resistin. Molecular and Cellular Biochemistry, 391(2), 117–125.
Dekang, L., Xiang, W., Jun, D., et al. (2016). Systematic characterization of lncRNAs’ cell-to-cell expression heterogeneity in glioblastoma cells. Oncotarget, 7(14), 18403–18414.
Bao, M. H., Luo, H. Q., Chen, L. H., et al. (2016). Impact of high fat diet on long non-coding RNAs and messenger RNAs expression in the aortas of ApoE(−/−) mice. Scientific Reports, 634161.
Lin, Z., Li, X., Zhan, X., et al. (2017). Construction of competitive endogenous RNA network reveals regulatory role of long non-coding RNAs in type 2 diabetes mellitus. Journal of Cellular and Molecular Medicine, 21(12), 3204–3213.
Jia, S., Yuting, R., Ming, W., et al. (2016). Differentially expressed circulating LncRNAs and mRNA identified by microarray analysis in obese patients. Scientific Reports, 635421.
Zhang, J., Zhang, N., Liu, M., et al. (2012). Disruption of growth factor receptor–binding protein 10 in the pancreas enhances β-cell proliferation and protects mice from Streptozotocin-induced β-cell apoptosis. Diabetes, 61(12), 3189.
Arimatsu, K., Yamada, H., Miyazawa, H., et al. (2014). Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Scientific Reports, 4(18), 4828.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods, 25(4), 402–408.
Zebda, N., Dubrovskyi, O., & Birukov, K. G. (2012). Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvascular Research, 83(1), 71–81.
Severs, N. J., Rothery, S., Dupont, E., et al. (2015). Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microscopy Research and Technique, 52(3), 301–322.
Ohanian, J., Pieri, M., & Ohanian, V. (2015). Non-receptor tyrosine kinases and the actin cytoskeleton in contractile vascular smooth muscle. Journal of Physiology, 593(17), 3807.
Undas, A., Stepień, E., Branicka, A., et al. (2009). Thrombin formation and platelet activation at the site of vascular injury in patients with coronary artery disease treated with clopidogrel combined with aspirin. Kardiologia Polska, 67(6), 591–598.
van Meeteren, L. A., Goumans, M. J., & Ten, D. P. (2011). TGF-β receptor signaling pathways in angiogenesis; emerging targets for anti-angiogenesis therapy. Current Pharmaceutical Biotechnology, 12(12), 2108–2120.
Schad, J. F., Meltzer, K. R., Hicks, M. R., et al. (2011). Cyclic strain upregulates VEGF and attenuates proliferation of vascular smooth muscle cells. Vasc Cell, 3(1), 21.
Oliver, C. J., Terrylorenzo, R. T., Elliott, E., et al. (2002). Targeting protein phosphatase 1 (PP1) to the actin cytoskeleton: the neurabin I/PP1 complex regulates cell morphology. Molecular & Cellular Biology, 22(13), 4690–4701.
Meyer, M. C., Rastogi, P., Beckett, C. S., et al. (2005). Phospholipase A2 inhibitors as potential anti-inflammatory agents. Current Pharmaceutical Design, 11(10), 1301–1312.
Martínezgarcía, C., Izquierdolahuerta, A., Vivas, Y., et al. (2015). Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS One, 10(11), e0142291.
Bharti, B., & Dey, C. S. (2008). Focal adhesion kinase contributes to insulin-induced actin reorganization into a mesh harboring glucose transporter-4 in insulin resistant skeletal muscle cells. BMC Cell Biology, 9(1), 1–12.
Mccullagh, K. A., & Balian, G. (1975). Collagen characterisation and cell transformation in human atherosclerosis. Nature, 258(5530), 73–75.
Faugeroux, J., Nematalla, H., Li, W., et al. (2013). Angiotensin II promotes thoracic aortic dissections and ruptures in Col3a1 haploinsufficient mice. Hypertension, 62(1), 203–208.
Oinuma, I., Kawada, K., Tsukagoshi, K., et al. (2012). Rnd1 and Rnd3 targeting to lipid raft is required for p190 RhoGAP activation. Molecular Biology of the Cell, 23, 1593.
Loirand, G., Cario-Toumaniantz, C., Chardin, P., et al. (1999). The rho-related protein Rnd1 inhibits Ca2+ sensitization of rat smooth muscle. The Journal of Physiology, 516(3), 825–834.
Chun, K. H., Araki, K., Jee, Y., et al. (2012). Regulation of glucose transport by ROCK1 differs from that of ROCK2 and is controlled by actin polymerization. Endocrinology, 153(4), 1649–1662.
Chen, M., Capps, C., Willerson, J. T., et al. (2002). E2F-1 regulates nuclear factor-kappaB activity and cell adhesion: potential antiinflammatory activity of the transcription factor E2F-1. Circulation, 106(21), 2707–2713.
Mzhavia, N., Yu, S., Ikeda, S., et al. (2008). Neuronatin: a new inflammation gene expressed on the aortic endothelium of diabetic mice. Diabetes, 57(10), 2774–2784.
Gburcik, V., Cleasby, M. E., & Timmons, J. A. (2013). Loss of neuronatin promotes “browning” of primary mouse adipocytes while reducing Glut1-mediated glucose disposal. American Journal of Physiology. Endocrinology and Metabolism, 304(8), E885–E894.
Carayol, J., Chabert, C., Cara, A. D., et al. (2017). Protein quantitative trait locus study in obesity during weight-loss identifies a leptin regulator. Nature Communications, 8(1), 2084.
Funding
This project was funded by the National Science Foundation for Young Scientists of China (grant number 81501955).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the Central South University Institute Animal Care and Use Committee.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Rights and permissions
About this article
Cite this article
Liu, S., Zheng, F., Cai, Y. et al. Effect of Long-Term Exercise Training on lncRNAs Expression in the Vascular Injury of Insulin Resistance. J. of Cardiovasc. Trans. Res. 11, 459–469 (2018). https://doi.org/10.1007/s12265-018-9830-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-018-9830-0