Abstract
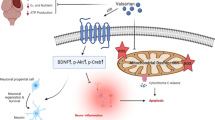
Metformin (MET), an antidiabetic agent, also has antioxidative effects in metabolic-related hypertension. This study was designed to determine whether MET has anti-hypertensive effects in salt-sensitive hypertensive rats by inhibiting oxidative stress in the hypothalamic paraventricular nucleus (PVN). Salt-sensitive rats received a high-salt (HS) diet to induce hypertension, or a normal-salt (NS) diet as control. At the same time, they received intracerebroventricular (ICV) infusion of MET or vehicle for 6 weeks. We found that HS rats had higher oxidative stress levels and mean arterial pressure (MAP) than NS rats. ICV infusion of MET attenuated MAP and reduced plasma norepinephrine levels in HS rats. It also decreased reactive oxygen species and the expression of subunits of NAD(P)H oxidase, improved the superoxide dismutase activity, reduced components of the renin-angiotensin system, and altered neurotransmitters in the PVN. Our findings suggest that central MET administration lowers MAP in salt-sensitive hypertension via attenuating oxidative stress, inhibiting the renin-angiotensin system, and restoring the balance between excitatory and inhibitory neurotransmitters in the PVN.








Similar content being viewed by others
References
Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens 1998, 16: 1979–1987.
de Wardener HE. The hypothalamus and hypertension. Physiol Rev 2001, 81: 1599–1658.
Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 1980, 31: 410–417.
Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007, 292: 82–97.
Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension 2010, 56: 325–330.
Braga VA, Medeiros IA, Ribeiro TP, Franca-Silva MS, Botelho-Ono MS, Guimaraes DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension. Braz J Med Biol Res 2011, 44: 871–876.
Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, Milner TA, et al. Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol Regul Integr Comp Physiol 2013, 304: 1096–1106.
Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol 2003, 139: 191–202.
Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertens Res 2011, 34: 1147–1160.
Kang YM, Yang Q, Yu XJ, Qi J, Zhang Y, Li HB, et al. Hypothalamic paraventricular nucleus activation contributes to neurohumoral excitation in rats with heart failure. Regen Med Res 2014, 2: 2.
Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, et al. Brain tumour necrosis factor-alpha modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res 2009, 83: 737–746.
Kang YM, Zhang AQ, Zhao XF, Cardinale JP, Elks C, Cao XM, et al. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res Cardiol 2011, 106: 473–483.
Nesti L, Natali A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr Metab Cardiovasc Dis 2017, 27: 657–669.
Gomez-Garcia A, Martinez Torres G, Ortega-Pierres LE, Rodriguez-Ayala E, Alvarez-Aguilar C. Rosuvastatin and metformin decrease inflammation and oxidative stress in patients with hypertension and dyslipidemia. Rev Esp Cardiol 2007, 60: 1242–1249.
He H, Zhao Z, Chen J, Ni Y, Zhong J, Yan Z, et al. Metformin-based treatment for obesity-related hypertension: a randomized, double-blind, placebo-controlled trial. J Hypertens 2012, 30: 1430–1439.
Tain YL, Wu KLH, Lee WC, Leu S, Chan JYH. Prenatal metformin therapy attenuates hypertension of developmental origin in male adult offspring exposed to maternal high-fructose and post-weaning high-fat diets. Int J Mol Sci 2018, 3: 19. pii: E1066.
Duan Q, Song P, Ding Y, Zou MH. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. Br J Pharmacol 2017, 174: 2140–2151.
Hamidi Shishavan M, Henning RH, van Buiten A, Goris M, Deelman LE, Buikema H. Metformin improves endothelial function and reduces blood pressure in diabetic spontaneously hypertensive rats independent from glycemia control: comparison to vildagliptin. Sci Rep 2017, 7: 10975.
Muntzel MS, Abe A, Petersen JS. Effects of adrenergic, cholinergic and ganglionic blockade on acute depressor responses to metformin in spontaneously hypertensive rats. J Pharmacol Exp Ther 1997, 281: 618–623.
Petersen JS, DiBona GF. Acute sympathoinhibitory actions of metformin in spontaneously hypertensive rats. Hypertension 1996, 27: 619–625.
Labuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopien B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep 2010, 62: 956–965.
Lv WS, Wen JP, Li L, Sun RX, Wang J, Xian YX, et al. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res 2012, 1444: 11–19.
Coleman BR, Carlezon WA Jr, Myers KM. Extinction of conditioned opiate withdrawal in rats is blocked by intracerebroventricular infusion of an NMDA receptor antagonist. Neurosci Lett 2013, 541: 39–42.
Petersen JS, Andersen D, Muntzel MS, Diemer NH, Holstein-Rathlou NH. Intracerebroventricular metformin attenuates salt-induced hypertension in spontaneously hypertensive rats. Am J Hypertens 2001, 14: 1116–1122.
Li HB, Qin DN, Ma L, Miao YW, Zhang DM, Lu Y, et al. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol Appl Pharmacol 2014, 279: 141–149.
Qi J, Yu XJ, Shi XL, Gao HL, Yi QY, Tan H, et al. NF-kappaB blockade in hypothalamic paraventricular nucleus inhibits high-salt-induced hypertension through NLRP3 and caspase-1. Cardiovasc Toxicol 2016, 16: 345–354.
Su Q, Huo CJ, Li HB, Liu KL, Li X, Yang Q, et al. Renin-angiotensin system acting on reactive oxygen species in paraventricular nucleus induces sympathetic activation via AT1R/PKCγ/Rac1 pathway in salt-induced hypertension. Sci Rep 2017, 7: 43107.
Li HB, Qin DN, Cheng K, Su Q, Miao YW, Guo J, et al. Central blockade of salusin β attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci Rep 2015, 5: 11162.
Su YT, Gu MY, Chu X, Feng X, Yu YQ. Whole-brain mapping of direct inputs to and axonal projections from GABAergic neurons in the parafacial zone. Neurosci Bull 2018, 34:485–496.
Zhang M, Qin DN, Suo YP, Su Q, Li HB, Miao YW, et al. Endogenous hydrogen peroxide in the hypothalamic paraventricular nucleus regulates neurohormonal excitation in high salt-induced hypertension. Toxicol Lett 2015, 235: 206–215.
Miller FJ, Jr Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res 1998, 82: 1298–1305.
Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 2014, 276: 115–120.
Rodrigo R, Gonzalez J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 2011, 34: 431–440.
Fujita M, Ando K, Nagae A, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in salt-sensitive hypertension. Hypertension 2007, 50: 360–367.
Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, et al. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 2012, 302: 1031–1049.
Gabor A, Leenen FH. Mechanisms mediating sodium-induced pressor responses in the PVN of Dahl rats. Am J Physiol Regul Integr Comp Physiol 2011, 301: 1338–1349.
Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res 2004, 95: 937–944.
Zhang Y, Yu Y, Zhang F, Zhong MK, Shi Z, Gao XY, et al. NAD(P)H oxidase in paraventricular nucleus contributes to the effect of angiotensin II on cardiac sympathetic afferent reflex. Brain Res 2006, 1082: 132–141.
Hernandez JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med 2014, 18: 709–720.
Mahrouf M, Ouslimani N, Peynet J, Djelidi R, Couturier M, Therond P, et al. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem Pharmacol 2006, 72: 176–183.
Jacintho JD, Kovacic P. Neurotransmission and neurotoxicity by nitric oxide, catecholamines, and glutamate: unifying themes of reactive oxygen species and electron transfer. Curr Med Chem 2003, 10: 2693–2703.
Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, et al. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci 2010, 30: 11317–11325.
Zhou FM, Cheng RX, Wang S, Huang Y, Gao YJ, Zhou Y, et al. Antioxidants attenuate acute and chronic itch: peripheral and central mechanisms of oxidative stress in pruritus. Neurosci Bull 2017, 33: 423–435.
Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci 2002, 22: 959–969.
Kang YM, Zhang DM, Yu XJ, Yang Q, Qi J, Su Q, et al. Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol 2014, 274: 436–444.
Biancardi VC, Campos RR, Stern JE. Altered balance of gamma-aminobutyric acidergic and glutamatergic afferent inputs in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus of renovascular hypertensive rats. J Comp Neurol 2010, 518: 567–585.
Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol 2006, 291: 2847–2856.
Martins-Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM. Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Front Physiol 2012, 3: 490.
Pavlov TS, Levchenko V, Ilatovskaya DV, Li H, Palygin O, Pastor-Soler NM, et al. Lack of effects of metformin and AICAR chronic infusion on the development of hypertension in Dahl salt-sensitive rats. Front Physiol 2017, 8: 227.
Acknowledgements
We gratefully acknowledge Jian-Jun Mu (Department of Cardiology, The First Affiliated Hospital of Xi’an Jiaotong University) for providing the Dahl salt-sensitive rats. This work was supported by the National Natural Science Foundation of China (81600333, 81770426, 81800372, 91439120, and 91639105), the Postdoctoral Science Foundation of China (2016M602835, 2017M620457), and the Postdoctoral Science Foundation of Shaanxi Province, China (2016BSHEDZZ91).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yu, XJ., Zhao, YN., Hou, YK. et al. Chronic Intracerebroventricular Infusion of Metformin Inhibits Salt-Sensitive Hypertension via Attenuation of Oxidative Stress and Neurohormonal Excitation in Rat Paraventricular Nucleus. Neurosci. Bull. 35, 57–66 (2019). https://doi.org/10.1007/s12264-018-0308-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-018-0308-5