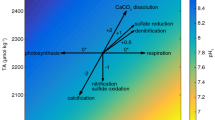
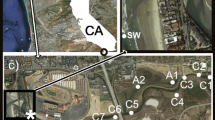
Abstract
It has been hypothesized that highly productive coastal ecosystems, such as seagrass meadows, could lead to the establishment of ocean acidification (OA) refugia, or areas of elevated pH and aragonite saturation state (Ωa) compared to source seawater. However, seagrass ecosystems experience extreme variability in carbonate chemistry across short temporal and small spatial scales, which could impact the pH buffering capacity of these potential refugia. Herein, short-term (hourly to diel) and small-scale (across 0.01–0.14 km2) spatiotemporal carbonate chemistry variability was assessed within two seagrass meadows in order to determine their short-term potential to elevate seawater pH relative to source seawater. Two locations at similar latitudes were chosen in order to compare systems dominated by coarse calcium carbonate (Bailey’s Bay, Bermuda) and muddy silicate (Mission Bay, CA, USA) sediments. In both systems, spatial variability of pH across the seagrass meadow at any given time was often greater than diel variability (e.g., the average range over 24 h) at any one site, with greater spatial variability occurring at low tide in Mission Bay. Mission Bay (spatial ΔpH = 0.08 ± 0.08; diel ΔpH = 0.12 ± 0.01; mean ± SD) had a greater average range in both temporal and spatial seawater chemistry than Bailey’s Bay (spatial ΔpH = 0.02 ± 0.01; diel ΔpH = 0.03 ± 0.00; mean ± SD). These differences were most likely due to a combination of slower currents, a larger tidal range, and more favorable weather conditions for photosynthesis (e.g., sunny with no rain) in Mission Bay. In both systems, there was a substantial amount of time (usually at night) when seawater pH within the seagrass beds was lower relative to the source seawater. Future studies aimed at assessing the potential of seagrass ecosystems to act as OA refugia for marine organisms need to account for the small-scale, high-frequency carbonate chemistry variability in both space and time, as this variability will impact where and when OA will be buffered or intensified.









Similar content being viewed by others
References
Andersson, A.J., K.L. Yeakel, N.R. Bates, and S.J. de Putron. 2014. Partial offsets in ocean acidification from changing coral reef biogeochemistry. Nature Climate Change 4 (1): 56–61.
Anthony, K., J.A. Kleypas, and J.P. Gattuso. 2011. Coral reefs modify their seawater carbon chemistry–implications for impacts of ocean acidification. Global Change Biology 17 (12): 3655–3666.
Bates, N.R., L. Samuels, and L. Merlivat. 2001. Biogeochemical and physical factors influencing seawater fCO2 and air-sea CO2 exchange on the Bermuda coral reef. Limnology and Oceanography 46 (4): 833–846.
Bates, N., Y. Astor, M. Church, K. Currie, J. Dore, M. Gonaález-Dávila, L. Lorenzoni, F. Muller-Karger, J. Olafsson, and M. Santa-Casiano. 2014. A Time-Series View of Changing Ocean Chemistry Due to Ocean Uptake of Anthropogenic CO2 and Ocean Acidification. Oceanography 27 (1): 126–141.
Bresnahan, P.J., T.R. Martz, Y. Takeshita, K.S. Johnson, and M. LaShomb. 2014. Best practices for autonomous measurement of seawater pH with the Honeywell Durafet. Methods in Oceanography 9: 44–60.
Bresnahan, P.J., T. Wirth, T.R. Martz, A.J. Andersson, T. Cyronak, S. D’Angelo, J. Pennise, W.K. Melville, L. Lenain, and N. Statom. 2016. A sensor package for mapping pH and oxygen from mobile platforms. Methods in Oceanography 17: 1–13.
Burdige, D.J., and R.C. Zimmerman. 2002. Impact of sea grass density on carbonate dissolution in Bahamian sediments. Limnology and Oceanography 47 (6): 1751–1763.
Burdige, D.J., R.C. Zimmerman, and X. Hu. 2008. Rates of carbonate dissolution in permeable sediments estimated from pore-water profiles: The role of sea grasses. Limnology and Oceanography 53 (2): 549–565.
Cai, W.J., Y. Wang, and R.E. Hodson. 1998. Acid-base properties of dissolved organic matter in the estuarine waters of Georgia, USA. Geochimica et Cosmochimica Acta 62 (3): 473–483.
Cai, W.-J., X. Hu, W.-J. Huang, M.C. Murrell, J.C. Lehrter, S.E. Lohrenz, W.-C. Chou, W. Zhai, J.T. Hollibaugh, and Y. Wang. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience 4 (11): 766–770.
Camp, E.F., D.J. Smith, C. Evenhuis, I. Enochs, D.P. Manzello, S. Woodcock, and D.J. Suggett. 2016a. Acclimatization to high-variance habitats does not enhance physiological tolerance of two key Caribbean corals to future temperature and pH. Proceedings of the Royal Society of London B: Biological Sciences 283 (1831): 20160442.
Camp, E.F., D.J. Suggett, G. Gendron, J. Jompa, C. Manfrino, and D.J. Smith. 2016b. Mangrove and seagrass beds provide different biogeochemical services for corals threatened by climate change. Frontiers in Marine Science 3: 52.
Challener, R.C., L.L. Robbins, and J.B. McClintock. 2016. Variability of the carbonate chemistry in a shallow, seagrass-dominated ecosystem: implications for ocean acidification experiments. Marine and Freshwater Research 67 (2): 163–172.
Clark, H.R., and C.J. Gobler. 2016. Diurnal fluctuations in CO2 and dissolved oxygen concentrations do not provide a refuge from hypoxia and acidification for early-life-stage bivalves. Marine Ecology Progress Series 558: 1–14.
Comeau, S., P.J. Edmunds, N.B. Spindel, and R.C. Carpenter. 2014. Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Marine Ecology Progress Series 501: 99–111.
Cyronak, T., I.R. Santos, D.V. Erler, D.T. Maher, and B.D. Eyre. 2014. Drivers of pCO2 variability in two contrasting coral reef lagoons: The influence of submarine groundwater discharge. Global Biogeochemical Cycles 28 (4): 398–414.
Davidson, C. 2015. Spatial and temporal variability of coastal carbonate chemistry in the Southern California region. La Jolla: Scripps Institution of Oceanography, University of California San Diego.
Dickson, A., and F. Millero. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Research Part A. Oceanographic Research Papers 34 (10): 1733–1743.
Dickson, A.G., C.L. Sabine, and J.R. Christian. 2007. Guide to best practices for ocean CO2 measurements. Sidney, British Columbia: North Pacific Marine Science Organization.
Doney, S.C., V.J. Fabry, R.A. Feely, and J.A. Kleypas. 2009. Ocean acidification: the other CO2 problem. Annual Review of Marine Science 1: 169–192.
Duarte, C.M. 1989. Temporal biomass variability and production/biomass relationships of seagrass communities. Marine Ecology Progress Series 51 (3): 269–276.
Duarte, C.M., J.J. Middelburg, and N. Caraco. 2005. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2 (1): 1–8.
Duarte, C.M., N. Marbà, E. Gacia, J.W. Fourqurean, J. Beggins, C. Barrón, and E.T. Apostolaki. 2010. Seagrass community metabolism: Assessing the carbon sink capacity of seagrass meadows. Global Biogeochemical Cycles 24 (4): GB4032.
Duarte, C.M., I.E. Hendriks, T.S. Moore, Y.S. Olsen, A. Steckbauer, L. Ramajo, J. Carstensen, J.A. Trotter, and M. McCulloch. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries and Coasts 36: 221–236.
Dufault, A.M., V.R. Cumbo, T.-Y. Fan, and P.J. Edmunds. 2012. Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proceedings of the Royal Society of London B: Biological Sciences 279 (1740): 2951–2958.
Fabricius, K.E., C. Langdon, S. Uthicke, C. Humphrey, S. Noonan, G. De'ath, R. Okazaki, N. Muehllehner, M.S. Glas, and J.M. Lough. 2011. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Climate Change 1 (3): 165–169.
Fourqurean, J.W., C.M. Duarte, H. Kennedy, N. Marba, M. Holmer, M.A. Mateo, E.T. Apostolaki, et al. 2012. Seagrass ecosystems as a globally significant carbon stock. Nature Geosci 5 (7): 505–509.
Gobler, C.J., H.R. Clark, A.W. Griffith, and M.W. Lusty. 2017. Diurnal Fluctuations in Acidification and Hypoxia Reduce Growth and Survival of Larval and Juvenile Bay Scallops (Argopecten irradians) and Hard Clams (Mercenaria mercenaria). Frontiers in Marine Science 3: 282.
Hall-Spencer, J.M., R. Rodolfo-Metalpa, S. Martin, E. Ransome, M. Fine, S.M. Turner, S.J. Rowley, D. Tedesco, and M.C. Buia. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454 (7200): 96–99.
Hendriks, I.E., Y.S. Olsen, L. Ramajo, L. Basso, A. Steckbauer, T.S. Moore, J. Howard, and C.M. Duarte. 2014. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11 (2): 333–346.
Holzer, K.K., and K.J. McGlathery. 2016. Cultivation grazing response in seagrass may depend on phosphorus availability. Marine Biology 163 (4): 1–11.
Johnson, M.R., S.L. Williams, C.H. Lieberman, and A. Solbak. 2003. Changes in the Abundance of the Seagrasses Zostera marina L. (Eelgrass) and Ruppia maritima L. (Widgeongrass) in San Diego, California, Following an El Nino Event. Estuaries 26 (1): 106–115.
Jury, C., F. Thomas, M. Atkinson, and R. Toonen. 2013. Buffer capacity, ecosystem feedbacks, and seawater chemistry under global change. Water 5 (3): 1303–1325.
Keppel, A.G., D.L. Breitburg, and R.B. Burrell. 2016. Effects of Co-Varying Diel-Cycling Hypoxia and pH on Growth in the Juvenile Eastern Oyster, Crassostrea virginica. PLoS ONE 11 (8): e0161088.
Kleypas, J.A., K.R.N. Anthony, and J.-P. Gattuso. 2011. Coral reefs modify their seawater carbon chemistry – Case study from a barrier reef (Moorea, French Polynesia). Global Change Biology 17 (12): 3667–3678.
Koch, M., G. Bowes, C. Ross, and X.-H. Zhang. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19 (1): 103–132.
Kroeker, K.J., R.L. Kordas, R.N. Crim, and G.G. Singh. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters 13 (11): 1419–1434.
Krumins, V., M. Gehlen, S. Arndt, P. Van Cappellen, and P. Regnier. 2013. Dissolved inorganic carbon and alkalinity fluxes from coastal marine sediments: model estimates for different shelf environments and sensitivity to global change. Biogeosciences 10 (1): 371–398.
Ku, T.C.W., L.M. Walter, M.L. Coleman, R.E. Blake, and A.M. Martini. 1999. Coupling between sulfur recycling and syndepositional carbonate dissolution: evidence from oxygen and sulfur isotope composition of pore water sulfate, South Florida Platform, U.S.A. Geochimica et Cosmochimica Acta 63 (17): 2529–2546.
Largier, J.L., J.T. Hollibaugh, and S.V. Smith. 1997. Seasonally Hypersaline Estuaries in Mediterranean-climate Regions. Estuarine, Coastal and Shelf Science 45 (6): 789–797.
Manzello, D.P., I.C. Enochs, N. Melo, D.K. Gledhill, and E.M. Johns. 2012. Ocean acidification refugia of the Florida reef tract. PLoS ONE 7 (7): e41715.
Martz, T.R., J.G. Connery, and K.S. Johnson. 2010. Testing the Honeywell Durafet® for seawater pH applications. Limnology and Oceanography: Methods 8 (5): 172–184.
Mateo, M.A., J. Cebrián, K. Dunton, and T. Mutchler. 2006. Carbon flux in seagrass ecosystems. Seagrasses: biology, ecology and conservation, 159–192. Dordrecht: Springer.
McLeod, E., G.L. Chmura, S. Bouillon, R. Salm, M. Björk, C.M. Duarte, C.E. Lovelock, W.H. Schlesinger, and B.R. Silliman. 2011. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers in Ecology and the Environment 9 (10): 552–560.
McLeod, E., K.R.N. Anthony, A. Andersson, R. Beeden, Y. Golbuu, J. Kleypas, K. Kroeker, et al. 2013. Preparing to manage coral reefs for ocean acidification: lessons from coral bleaching. Frontiers in Ecology and the Environment 11 (1): 20–27.
Mehrbach, C., C. Culberson, J. Hawley, and R. Pytkowicz. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnology and Oceanography 18 (6): 897–907.
Millero, F.J., W.T. Hiscock, F. Huang, M. Roche, and J.Z. Zhang. 2001. Seasonal variation of the carbonate system in Florida Bay. Bulletin of Marine Science 68 (1): 101–123.
Mongin, M., M.E. Baird, S. Hadley, and A. Lenton. 2016. Optimising reef-scale CO2 removal by seaweed to buffer ocean acidification. Environmental Research Letters 11 (3): 034023.
Nellemann, C., and E. Corcoran. 2009. Blue carbon: the role of healthy oceans in binding carbon: a rapid response assessment. Norway: United Nations Environment Programme, GRID-Arendal.
Orr, J.C., V.J. Fabry, O. Aumont, L. Bopp, S.C. Doney, R.A. Feely, A. Gnanadesikan, N. Gruber, A. Ishida, and F. Joos. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437 (7059): 681–686.
Ow, Y.X., C.J. Collier, and S. Uthicke. 2015. Responses of three tropical seagrass species to CO2 enrichment. Marine Biology 162 (5): 1005–1017.
Palacios, S.L., and R.C. Zimmerman. 2007. Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Marine Ecology Progress Series 344: 1–13.
Price, N.N., T.R. Martz, R.E. Brainard, and J.E. Smith. 2012. Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PLoS ONE 7 (8): e43843.
Rasheed, M., C. Wild, U. Franke, and M. Huettel. 2004. Benthic photosynthesis and oxygen consumption in permeable carbonate sediments at Heron Island, Great Barrier Reef, Australia. Estuarine, Coastal and Shelf Science 59 (1): 139–150.
Rivest, E.B., S. Comeau, and C.E. Cornwall. 2017. The Role of Natural Variability in Shaping the Response of Coral Reef Organisms to Climate Change. Current Climate Change Reports 3 (4): 271–281.
Santos, I.R., B.D. Eyre, and M. Huettel. 2012. The driving forces of porewater and groundwater flow in permeable coastal sediments: A review. Estuarine, Coastal and Shelf Science 98: 1–15.
Semesi, I.S., S. Beer, and M. Björk. 2009. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Marine Ecology Progress Series 382: 41–47.
Shaw, E.C., B.I. McNeil, and B. Tilbrook. 2012. Impacts of ocean acidification in naturally variable coral reef flat ecosystems. Journal of Geophysical Research 117: C03038.
Shaw, E.C., B.I. McNeil, B. Tilbrook, R. Matear, and M.L. Bates. 2013. Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Global Change Biology 19 (5): 1632–1641.
Sippo, J.Z., D.T. Maher, D.R. Tait, C. Holloway, and I.R. Santos. 2016. Are mangroves drivers or buffers of coastal acidification? Insights from alkalinity and dissolved inorganic carbon export estimates across a latitudinal transect. Global Biogeochemical Cycles 30: 753–766.
Takeshita, Y., W. McGillis, E.M. Briggs, A.L. Carter, E.M. Donham, T.R. Martz, N.N. Price, and J.E. Smith. 2016. Assessment of net community production and calcification of a coral reef using a boundary layer approach. Journal of Geophysical Research: Oceans. 121: 5655–5671.
Turk, D., K. Yates, M. Vega-Rodriguez, G. Toro-Farmer, C. L'Esperance, N. Melo, D. Ramsewak, et al. 2015. Community metabolism in shallow coral reef and seagrass ecosystems, Lower Florida Keys. Marine Ecology Progress Series 538: 35–52.
Unsworth, R.K.F., C.J. Collier, G.M. Henderson, and L.J. McKenzie. 2012. Tropical seagrass meadows modify seawater carbon chemistry: implications for coral reefs impacted by ocean acidification. Environmental Research Letters 7 (2): 024026.
Van Heuven, S., D. Pierrot, J. Rae, E. Lewis, and D. Wallace. 2011. CO2SYS v 1.1, MATLAB program developed for CO2 system calculations. ORNL/CDIAC-105b, Carbon Dioxide Inf. Anal. Center, Oak Ridge Natl. Lab. US DoE, doi 10.
Wild, C., M.S. Naumann, A. Haas, U. Struck, F.W. Mayer, M.Y. Rasheed, and M. Huettel. 2009. Coral sand O2 uptake and pelagic–benthic coupling in a subtropical fringing reef, Aqaba, Red Sea. Aquatic Biology 6: 133–142.
Zimmerman, R.C., D.G. Kohrs, D.L. Steller, and R.S. Alberte. 1997. Impacts of CO2 Enrichment on Productivity and Light Requirements of Eelgrass. Plant Physiology 115 (2): 599–607.
Zimmerman, R.C., V.J. Hill, and C.L. Gallegos. 2015. Predicting effects of ocean warming, acidification, and water quality on Chesapeake region eelgrass. Limnology and Oceanography 60 (5): 1781–1804.
Zimmerman, R.C., V.J. Hill, M. Jinuntuya, B. Celebi, D. Ruble, M. Smith, T. Cedeno, and W.M. Swingle. 2017. Experimental impacts of climate warming and ocean carbonation on eelgrass Zostera marina. Marine Ecology Progress Series 566: 1–15.
Acknowledgements
The authors would like to thank the Ocean Discovery Institute (ODI) and over 30 middle school students from the City Heights area in San Diego who helped collect data in Mission Bay. We would also like to thank the staff of the Bermuda Institute of Ocean Sciences (BIOS) for their support during the Bailey’s Bay study. This research was part of NSF grant OCE 12-55042 (AJA).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Margaret R. Mulholland
Rights and permissions
About this article
Cite this article
Cyronak, T., Andersson, A.J., D’Angelo, S. et al. Short-Term Spatial and Temporal Carbonate Chemistry Variability in Two Contrasting Seagrass Meadows: Implications for pH Buffering Capacities. Estuaries and Coasts 41, 1282–1296 (2018). https://doi.org/10.1007/s12237-017-0356-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0356-5