Abstract
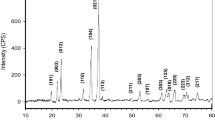
Self-made enriched 10B boric acid as raw material was purified by recrystallization. The effects of final crystallization temperature, crystallization time, stirring speed, crystallization frequency and other factors on the purity were investigated. The appropriate operating condition was that the final crystallization temperature and time were 5°C and 10 h respectively under a low-speed stirring for crystallizing twice, which would make the purity and yield of boric acid reach 99.94% and 95.36%, respectively. Taking this as foundation, recrystallization process was optimized with acetone as anti-solvent, whose amount was the most important index. The boric acid solution was added into acetone and recrystallized under the same condition, and the purity and yield of boric acid would reach 99.98% and 99.61%, respectively. The product detected by XRD was confirmed as boric acid crystal. Main ion concentration in the product was detected by ICP, which basically met the national standard of high purity. Crystal morphology of boric acid was observed by SEM.
Similar content being viewed by others
References
Wang Yongpeng, Liu Mengzhu, Zhang Yuxuan et al. Synthesis and properties of a novel boron-containing poly(arylether ketone)and its application in the preparation of azo poly(aryl ether ketone)[J]. Chem J Chinese Universities, 2014, 35(5): 1086–1092(in Chinese).
Xue Yunna, Li Jiaoyi, Hao Zhijun et al. Synthesis, thermal decomposition mechanism and kinetic equation of polyhedral boron hydride compound [(C2H5)4N]2B10H10 with high enthalpy of combustion[J]. Chem J Chinese Universities, 2015, 36(2): 375–380(in Chinese).
Zhang Lei, Zhang Weijiang, Xu Jiao et al. Synthesis of enriched 10B boric acid of nuclear grade[J]. Transactions of Tianjin University, 2014, 20(6): 458–462.
Zhang Lei, Zhang Weijiang, Xu Jiao et al. Preparation of boric-10 acid applied in nuclear industry[J]. Transactions of Tianjin University, 2015, 21(2): 172–177.
Zhang Jian. Application of chemical(boron concentration) regulation in pressurized water reactor in nuclear power station[J]. Nuclear Power Engineering, 1982, 3(5): 85–89(in Chinese).
Han Liguo, Yu Jingyang, Zhang Weijiang. The enrichment technology for the separation and production of boron isotopes[J]. Journal of Isotope, 2006, 19(1): 47–52(in Chinese).
Zhang Weijiang, Han Liguo. Application of enriched boron-10 in nuclear energy [C]. In: Symposium on Isotope Technology and Application Conference. Beijing Nuclear Society, Beijing, 2005: 44–49(in Chinese).
Zheng Xuejia. Production and Application of Boron Compounds[M]. Chemical Industry Press, Beijing, 2008(in Chinese).
Debora N, Carles T, Ricard G et al. Purification of xylooligosaccharides from almond shells by ultrafiltration[J]. Separation Purification Technology, 2007, 53: 235–243.
Yan Mingfang, Qiu Xueqing, Yang Dongjie et al. Separation and purification of lignin sulfonate[J]. Chem J Chinese Universities, 2008, 29(11): 2312–2316(in Chinese).
Ramachandra Murty B. A simple and illustrative method of iron-exchange chromatography[J]. Resonance, 2003, 8(12): 77–82.
Qian Tingbao. Applications of Ion Exchange Technology [M]. Tianjin Science and Technology Press, Tianjin, 1984(in Chinese).
Ning Guiling, Ye Junwei, Pan Xin’ai et al. A method of complex crystallization method to remove the metal impurities in boric acid: CP101575100A[P]. 2009-11-11.
Sahin Ö, Özdemir M, Genli N. Effect of impurities on crystal growth rate of ammonium pentaborate [J]. Journal of Crystal Growth, 2004, 260(1/2): 223–231.
Sahin Ö, Bulutcu A N. Effect of surface charge distribution on the crystal growth of sodium perborate tetrahydrate[J]. Journal of Crystal Growth, 2002, 241(4): 471–480.
Bennema P, Mullin J W. Industrial Crystallization[M]. Plenum Press, New York, 1986.
Bell H, Kimber W L, Li M et al. Liposomal transfection efficiency and toxicity on glioma cell lines: In vitro and in vivo studies[J]. Neuroreport, 1998, 9(5): 793–798.
Mehta S C, Lu D R. Targeted drug delivery for boron neutron capture therapy[J]. Pharm Res, 1996, 13: 344–351.
Ministry of Chemical Industry. Chemical Reagent Standard[ M]. Chemical Industry Press, Beijing, 1995(in Chinese).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Liu, T. & Xu, J. Preparation and characterization of high purity enriched 10B boric acid via anti-solvent recrystallization. Trans. Tianjin Univ. 22, 279–283 (2016). https://doi.org/10.1007/s12209-016-2697-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-016-2697-8