Abstract
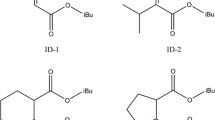
A series of electron donors, including 1,1-cyclopentanecarboxylic acid diethyl ester (CPCADEE), 1,1-cyclopentanedimethanol acetic diester (CPDMAD), 1,1-biethoxymethyl pentane (BEMP), 2,2-diethyl diethylmalonate (DEDEM)and 2,2-diethyl-1,3-propanediol acetic diester (DEPDADE), were synthesized by diethyl malonate (DEM). The purities and structures of the above products were characterized by gas chromatography (GC) and gas chromatography-mass spectrometer (GC-MS), respectively. Furthermore, the possible optimal three-dimensional structures of these donors were simulated by means of Gaussian 03 and Chem 3D. Then these electron donors were coordinated with tetrachloro titanium (TiCl4) and chloride magnesium (MgCl2) to obtain the catalysts for the polymerization of propylene. The catalytic activities and properties of polypropylene are greatly improved by adding external donor(ED) when CPCADEE or DEPDADE is used as internal donor(ID). However, when BEMP was used as ID, the highest catalytic activity is obtained without adding ED, which can reduce production costs and simplify catalytic synthesis. The experiments indicate that BEMP has the shortest distance of oxygen atoms and the highest electronegativity.
Similar content being viewed by others
References
Kaminsky W. Highly active metallocene catalysts for olefin polymerization[J]. Journal of the Chemical Society-Dalton Transactions, 1998(9): 1413–1418.
Galli P, Vecellio G. Technology: Driving force behind innovation and growth of polyolefins[J]. Progress in Polymer Science, 2001, 26(8): 1287–1336.
Paolo C. The discovery of isotactic polypropylene and its impact on pure and applied science[J]. Journal of Polymer Science-Part A: Polymer Chemistry, 2004, 42(3): 391–395.
Kashiwa N. The discovery and progress of MgC12-supported TiCl4 catalysts[J]. Journal of Polymer Science-Part A: Polymer Chemistry, 2004, 42(1): 1–8.
Albizzati E, Galimberti M. Catalysts for olefins polymerization[ J]. Catalysis Today, 1998, 41(1–3): 159–168.
Ewen J A. Mechanisms of stereochemical control in propylene polymerization with soluble Group 4B metallocene/methylalumoxane catalysts[J]. Journal of the American Chemical Society, 1984, 106(21): 6355–6364.
Sacchi M C, Forlini F, Tritto I et al. Stereochemistry of the initiation step in Ziegler-Natta catalysts containing dialkyl propane diethers: A tool for distinguishing the role of internal and external donors[J]. Macromolecular Symposia, 1995, 89(1): 91–100.
Cecchin G, Morini G, Pelliconi A. Polypropene product innovation by reactor granule technology[J]. Macromolecular Symposia, 2001, 173(1): 195–210.
Potapov A G, Bukatov G D, Zakharov V A. DRIFT study of internal donors in supported Ziegler-Natta catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2006, 246(1/2): 248–254.
Zohuri G H, Kasaeian A B, Angagi M T et al. Polymerization of propylene using MgCl2(ethoxide type)/TiCl4/diether heterogeneous Ziegler-Natta catalyst[J]. Polymer International, 2005, 54(6): 882–885.
Xu D M, Liu Z Y, Zhao J et al. Highly active MgCl2-supported catalysts containing novel diether donors for propylene polymerization[J]. Macromolecular Rapid Communications, 2000, 21(15): 1046–1049.
Zhong C F, Gao M Z, Mao B Q. Effect of Et3Al and 9,9-bis(methoxymethyl)fluorine on propylene polymerization at high temperature with TiCl4/MgCl2 catalysts[J]. Macromolecular Chemistry and Physics, 2005, 206(3): 404–409.
Chen Zhikun. Synthesis of Donor and Its Effect on Performance of MgCl2/TiCl4/ID Catalysts for Propylene Polymerization[ D]. School of Chemical Engineering and Technology, Tianjin University, Tianjin, 2008(in Chinese).
Kazuo Soga, Takeshi Shiono, Yoshiharu Doi. Influence of internal and external donors on activity and stereospecificity of Ziegler-Natta catalysts[J]. Macromolecular Chemistry and Physics, 1988, 189(7): 1531–1541.
Hideharu Mori, Hiromitsu Iguchi, Kouichi Hasebe et al. Kinetic study of isospecific active sites formed by various alkylaluminiums on MgCl2-supported Ziegler catalyst at the initial stage of propene polymerization[J]. Macromolecular Chemistry and Physics, 1997, 198(4): 1249–1255.
Kemp R A, Brown D S, Lattman M et al. Calixarenes as a new class of external electron donors in Ziegler-Natta polypropylene catalysts[J]. Journal of Molecular Catalysis A: Chemical, 1999, 149(1/2): 125–133.
Sacchi M C, Tritto I, Locatelli P. Setreochemical investigation of the effect of lewis bases in heterogeneous Ziegler-Natta initiator systems[J]. Progress in Polymer Science, 1991, 16(2/3): 331–360.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Natural Science Foundation of China (No. 20476080) and Tianjin Natural Science Foundation (No. 07JCYBJC00600).
GUO Jintang, born in 1968, female, Dr, associate Prof.
Rights and permissions
About this article
Cite this article
Guo, J., Hu, G. & Chen, Z. Synthesis of novel electron donors and their application to propylene polymerization. Trans. Tianjin Univ. 18, 8–14 (2012). https://doi.org/10.1007/s12209-012-1805-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-012-1805-7