Abstract
Introduction
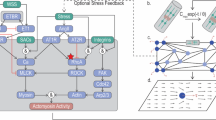
In response to external stress, cells alter their morphology, metabolic activity, and functions to mechanically adapt to the dynamic, local environment through cell allostasis. To explore mechanotransduction in cellular allostasis, we applied an integrated micromechanical system that combines an ‘ultrasound tweezers’-based mechanical stressor and a Förster resonance energy transfer (FRET)-based molecular force biosensor, termed “actinin-sstFRET,” to monitor in situ single-cell allostasis in response to transient stimulation in real time.
Methods
The ultrasound tweezers utilize 1 Hz, 10-s transient ultrasound pulses to acoustically excite a lipid-encapsulated microbubble, which is bound to the cell membrane, and apply a pico- to nano-Newton range of forces to cells through an RGD-integrin linkage. The actinin-sstFRET molecular sensor, which engages the actin stress fibers in live cells, is used to map real-time actomyosin force dynamics over time. Then, the mechanosensitive behaviors were examined by profiling the dynamics in Ca2+ influx, actomyosin cytoskeleton (CSK) activity, and GTPase RhoA signaling to define a single-cell mechanical allostasis.
Results
By subjecting a 1 Hz, 10-s physical stress, single vascular smooth muscle cells (VSMCs) were observed to remodeled themselves in a biphasic mechanical allostatic manner within 30 min that caused them to adjust their contractility and actomyosin activities. The cellular machinery that underscores the vital role of CSK equilibrium in cellular mechanical allostasis, includes Ca2+ influx, remodeling of actomyosin CSK and contraction, and GTPase RhoA signaling. Mechanical allostasis was observed to be compromised in VSMCs from patients with type II diabetes mellitus (T2DM), which could potentiate an allostatic maladaptation.
Conclusions
By integrating tools that simultaneously permit localized mechanical perturbation and map actomyosin forces, we revealed distinct cellular mechanical allostasis profiles in our micromechanical system. Our findings of cell mechanical allostasis and maladaptation provide the potential for mechanophenotyping cells to reveal their pathogenic contexts and their biophysical mediators that underlie multi-etiological diseases such as diabetes, hypertension, or aging.





Similar content being viewed by others
References
Alhussein, G., A. Shanti, I. A. Farhat, S. B. Timraz, N. S. Alwahab, Y. E. Pearson, M. N. Martin, N. Christoforou, and J. C. Teo. A spatiotemporal characterization method for the dynamic cytoskeleton. Cytoskeleton 73:221–232, 2016.
Allingham, J. S., R. Smith, and I. Rayment. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 12:378, 2005.
Balasubramanian, L., C.-M. Lo, J. S. Sham, and K.-P. Yip. Remanent cell traction force in renal vascular smooth muscle cells induced by integrin-mediated mechanotransduction. Am. J. Physiol. Cell Physiol. 304:C382–C391, 2013.
Beningo, K. A., K. Hamao, M. Dembo, Y.-L. Wang, and H. Hosoya. Traction forces of fibroblasts are regulated by the Rho-dependent kinase but not by the myosin light chain kinase. Arch. Biochem. Biophys. 456:224–231, 2006.
Binnewies, M., E. W. Roberts, K. Kersten, V. Chan, D. F. Fearon, M. Merad, L. M. Coussens, D. I. Gabrilovich, S. Ostrand-Rosenberg, and C. C. Hedrick. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24:541–550, 2018.
Blanchoin, L., R. Boujemaa-Paterski, C. Sykes, and J. Plastino. Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94:235–263, 2014.
Bonakdar, N., R. Gerum, M. Kuhn, M. Spörrer, A. Lippert, W. Schneider, K. E. Aifantis, and B. Fabry. Mechanical plasticity of cells. Nat. Mater. 15:1090, 2016.
Broussard, J. A., B. Rappaz, D. J. Webb, and C. M. Brown. Fluorescence resonance energy transfer microscopy as demonstrated by measuring the activation of the serine/threonine kinase Akt. Nat. Protoc. 8:265, 2013.
Brown, R., R. Prajapati, D. McGrouther, I. Yannas, and M. Eastwood. Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 175:323–332, 1998.
Chen, W., S. G. Allen, W. Qian, Z. Peng, S. Han, X. Li, Y. Sun, C. Fournier, L. Bao, and R. H. Lam. Biophysical phenotyping and modulation of ALDH + inflammatory breast cancer stem-like cells. Small 15:1802891, 2019.
Chen, D., Y. Sun, C. X. Deng, and J. Fu. Improving survival of disassociated human embryonic stem cells by mechanical stimulation using acoustic tweezing cytometry. Biophys. J. 108:1315–1317, 2015.
Chen, D., Y. Sun, M. S. Gudur, Y.-S. Hsiao, Z. Wu, J. Fu, and C. X. Deng. Two-bubble acoustic tweezing cytometry for biomechanical probing and stimulation of cells. Biophys. J. 108:32–42, 2015.
Chowdhury, F., S. Na, D. Li, Y.-C. Poh, T. S. Tanaka, F. Wang, and N. Wang. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 9:82, 2010.
Chrzanowska-Wodnicka, M., and K. Burridge. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 133:1403–1415, 1996.
Collinsworth, A. M., C. E. Torgan, S. N. Nagda, R. J. Rajalingam, W. E. Kraus, and G. A. Truskey. Orientation and length of mammalian skeletal myocytes in response to a unidirectional stretch. Cell Tissue Res. 302:243–251, 2000.
Coste, B., B. Xiao, J. S. Santos, R. Syeda, J. Grandl, K. S. Spencer, S. E. Kim, M. Schmidt, J. Mathur, and A. E. Dubin. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483:176, 2012.
Dietzel, I., and U. Heinemann. Dynamic variations of the brain cell microenvironment in relation to neuronal hyperactivity. Ann. N. Y. Acad. Sci. 481:72–84, 1986.
Du Roure, O., A. Saez, A. Buguin, R. H. Austin, P. Chavrier, P. Siberzan, and B. Ladoux. Force mapping in epithelial cell migration. Proc. Natl. Acad. Sci. USA 102:2390–2395, 2005.
Fan, Z., Y. Sun, D. Chen, D. Tay, W. Chen, C. X. Deng, and J. Fu. Acoustic tweezing cytometry for live-cell subcellular modulation of intracellular cytoskeleton contractility. Sci. Rep. 3:2176, 2013.
Fan, Z., X. Xue, R. Perera, S. Nasr Esfahani, A. A. Exner, J. Fu, and C. X. Deng. Acoustic actuation of integrin-bound microbubbles for mechanical phenotyping during differentiation and morphogenesis of human embryonic stem cells. Small 14:1803137, 2018.
Faust, U., N. Hampe, W. Rubner, N. Kirchgessner, S. Safran, B. Hoffmann, and R. Merkel. Cyclic stress at mHz frequencies aligns fibroblasts in direction of zero strain. PLoS ONE 6:e28963, 2011.
Fu, J., Y.-K. Wang, M. T. Yang, R. A. Desai, X. Yu, Z. Liu, and C. S. Chen. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 7:733, 2010.
Gattazzo, F., A. Urciuolo, and P. Bonaldo. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2506–2519:2014, 1840.
Ghigo, A., M. Laffargue, M. Li, and E. Hirsch. PI3 K and calcium signaling in cardiovascular disease. Circ. Res. 121:282–292, 2017.
Goldstein, D. S., and B. McEwen. Allostasis, homeostats, and the nature of stress. Stress 5:55–58, 2002.
Gunst, S. J., and W. Zhang. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 295:C576–C587, 2008.
Hayakawa, K., H. Tatsumi, and M. Sokabe. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J. Cell Sci. 121:496–503, 2008.
Heureaux, J., D. Chen, V. L. Murray, C. X. Deng, and A. P. Liu. Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell. Mol. Bioeng. 7:307–319, 2014.
Hoffman, B. D., C. Grashoff, and M. A. Schwartz. Dynamic molecular processes mediate cellular mechanotransduction. Nature 475:316, 2011.
Hsu, H.-J., C.-F. Lee, and R. Kaunas. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS ONE 4:e4853, 2009.
Humphrey, J. D., E. R. Dufresne, and M. A. Schwartz. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15:802, 2014.
Kaksonen, M., C. P. Toret, and D. G. Drubin. A modular design for the clathrin-and actin-mediated endocytosis machinery. Cell 123:305–320, 2005.
Kato, S., T. Osa, and T. Ogasawara. Kinetic model for isometric contraction in smooth muscle on the basis of myosin phosphorylation hypothesis. Biophys. J. 46:35, 1984.
Kaunas, R., P. Nguyen, S. Usami, and S. Chien. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA 102:15895–15900, 2005.
Kim, T.-J., C. Joo, J. Seong, R. Vafabakhsh, E. L. Botvinick, M. W. Berns, A. E. Palmer, N. Wang, T. Ha, and E. Jakobsson. Distinct mechanisms regulating mechanical force-induced Ca2+ signals at the plasma membrane and the ER in human MSCs. Elife 4:e04876, 2015.
Kranenburg, O., M. Poland, M. Gebbink, L. Oomen, and W. H. Moolenaar. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J. Cell Sci. 110:2417–2427, 1997.
Labernadie, A., T. Kato, A. Brugués, X. Serra-Picamal, S. Derzsi, E. Arwert, A. Weston, V. González-Tarragó, A. Elosegui-Artola, and L. Albertazzi. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19:224, 2017.
Lam, R. H., Y. Sun, W. Chen, and J. Fu. Elastomeric microposts integrated into microfluidics for flow-mediated endothelial mechanotransduction analysis. Lab Chip 12:1865–1873, 2012.
Livne, A., E. Bouchbinder, and B. Geiger. Cell reorientation under cyclic stretching. Nat. Commun. 5:3938, 2014.
Mann, J. M., R. H. Lam, S. Weng, Y. Sun, and J. Fu. A silicone-based stretchable micropost array membrane for monitoring live-cell subcellular cytoskeletal response. Lab Chip 12:731–740, 2012.
McEwen, B. S. Stress, adaptation, and disease: allostasis and allostatic load. Ann. N. Y. Acad. Sci. 840:33–44, 1998.
McEwen, B. S., and J. C. Wingfield. The concept of allostasis in biology and biomedicine. Horm. Behav. 43:2–15, 2003.
Meng, F., and F. Sachs. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J. Cell Sci. 124:261–269, 2011.
Milewicz, D. M., K. M. Trybus, D.-C. Guo, H. L. Sweeney, E. Regalado, K. Kamm, and J. T. Stull. Altered smooth muscle cell force generation as a driver of thoracic aortic aneurysms and dissections. Arterioscler. Thromb. Vasc. Biol. 37:26–34, 2017.
Murrell, M., P. W. Oakes, M. Lenz, and M. L. Gardel. Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16:486, 2015.
Palmer, A. E., and R. Y. Tsien. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat. Protoc. 1:1057, 2006.
Pasterkamp, G., D. P. de Kleijn, and C. Borst. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc. Res. 45:843–852, 2000.
Porter, K. E., and K. Riches. The vascular smooth muscle cell: a therapeutic target in type 2 diabetes? Clin. Sci. 125:167–182, 2013.
Pyle, A. L., and P. P. Young. Atheromas feel the pressure: biomechanical stress and atherosclerosis. Am. J. Pathol. 177:4–9, 2010.
Qian, W., L. Gong, X. Cui, Z. Zhang, A. Bajpai, C. Liu, A. B. Castillo, J. C. Teo, and W. Chen. Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl. Mater. Interfaces. 9:41794–41806, 2017.
Raftopoulou, M., and A. Hall. Cell migration: Rho GTPases lead the way. Dev. Biol. 265:23–32, 2004.
Rahimzadeh, J., F. Meng, F. Sachs, J. Wang, D. Verma, and S. Z. Hua. Real-time observation of flow-induced cytoskeletal stress in living cells. Am. J. Physiol. Cell Physiol. 301:C646–C652, 2011.
Retailleau, K., F. Duprat, M. Arhatte, S. S. Ranade, R. Peyronnet, J. R. Martins, M. Jodar, C. Moro, S. Offermanns, and Y. Feng. Piezo1 in smooth muscle cells is involved in hypertension-dependent arterial remodeling. Cell Rep. 13:1161–1171, 2015.
Riches, K., P. Warburton, D. J. O’Regan, N. A. Turner, and K. E. Porter. Type 2 diabetes impairs venous, but not arterial smooth muscle cell function: possible role of differential RhoA activity. Cardiovasc. Revasc. Med. 15:141–148, 2014.
Ritsma, L., S. I. Ellenbroek, A. Zomer, H. J. Snippert, F. J. de Sauvage, B. D. Simons, H. Clevers, and J. van Rheenen. Intestinal crypt homeostasis revealed at single-stem-cell level by in vivo live imaging. Nature 507:362, 2014.
Schwartz, M. W., R. J. Seeley, M. H. Tschöp, S. C. Woods, G. J. Morton, M. G. Myers, and D. D’alessio. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503:59, 2013.
Shao, Y., J. M. Mann, W. Chen, and J. Fu. Global architecture of the F-actin cytoskeleton regulates cell shape-dependent endothelial mechanotransduction. Integr. Biol. 6:300–311, 2014.
Shyu, K.-G. Cellular and molecular effects of mechanical stretch on vascular cells and cardiac myocytes. Clin. Sci. 116:377–389, 2009.
Sukharev, S., M. Betanzos, C.-S. Chiang, and H. R. Guy. The gating mechanism of the large mechanosensitive channel MscL. Nature 409:720, 2001.
Sun, Z., S. S. Guo, and R. Fässler. Integrin-mediated mechanotransduction. J. Cell Biol. 215:445–456, 2016.
Topal, T., X. Hong, X. Xue, Z. Fan, N. Kanetkar, J. T. Nguyen, J. Fu, C. X. Deng, and P. H. Krebsbach. Acoustic tweezing cytometry induces rapid initiation of human embryonic stem cell differentiation. Sci. Rep. 8:12977, 2018.
Waddingham, M. T., A. J. Edgley, H. Tsuchimochi, D. J. Kelly, M. Shirai, and J. T. Pearson. Contractile apparatus dysfunction early in the pathophysiology of diabetic cardiomyopathy. World J. Diabetes 6:943, 2015.
Wang, Y., E. L. Botvinick, Y. Zhao, M. W. Berns, S. Usami, R. Y. Tsien, and S. Chien. Visualizing the mechanical activation of Src. Nature 434:1040, 2005.
Wang, Y., J. Y.-J. Shyy, and S. Chien. Fluorescence proteins, live-cell imaging, and mechanobiology: seeing is believing. Annu. Rev. Biomed. Eng. 10:1–38, 2008.
Wang, Y., and N. Wang. FRET and mechanobiology. Integr. Biol. 1:565–573, 2009.
Webster, K. D., W. P. Ng, and D. A. Fletcher. Tensional homeostasis in single fibroblasts. Biophys. J. 107:146–155, 2014.
Weng, S., Y. Shao, W. Chen, and J. Fu. Mechanosensitive subcellular rheostasis drives emergent single-cell mechanical homeostasis. Nat. Mater. 15:961, 2016.
Xue, X., X. Hong, Z. Li, C. X. Deng, and J. Fu. Acoustic tweezing cytometry enhances osteogenesis of human mesenchymal stem cells through cytoskeletal contractility and YAP activation. Biomaterials 134:22–30, 2017.
Xue, X., Y. Sun, A. M. Resto-Irizarry, Y. Yuan, K. M. A. Yong, Y. Zheng, S. Weng, Y. Shao, Y. Chai, and L. Studer. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 17:633–641, 2018.
Yang, M. T., J. Fu, Y.-K. Wang, R. A. Desai, and C. S. Chen. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat. Protoc. 6:187, 2011.
Yang, M. T., D. H. Reich, and C. S. Chen. Measurement and analysis of traction force dynamics in response to vasoactive agonists. Integr. Biol. 3:663–674, 2011.
Zhang, Y., A. Gordon, W. Qian, and W. Chen. Engineering nanoscale stem cell niche: Direct stem cell behavior at cell–matrix interface. Adv. Healthc. Mater. 4:1900–1914, 2015.
Acknowledgments
We acknowledge financial support from the Department of Mechanical and Aerospace Engineering at New York University, the American Heart Association Scientist Development Grant (16SDG31020038), the National Science Foundation (CBET 1701322), and the National Institute of Health (R21EB025406).
Conflict of interest
Weiyi Qian and Weiqiang Chen declare that they have no conflicts of interest.
Ethical Approval
This study does not involve any human studies and animal studies by any author in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stephanie Michelle Willerth oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weiqiang Chen is an Assistant Professor in the Departments of Mechanical and Aerospace Engineering and Biomedical Engineering at New York University. He received his B.S. in Physics from Nanjing University and M.S. degrees from Shanghai Jiao Tong University and Purdue University, both in Electrical Engineering. He earned his Ph.D. in Mechanical Engineering from the University of Michigan in 2014. Research in the Chen’s lab focuses on developing new biomaterials, microfluidics, and organ-on-a-chip systems to address emerging biomedical problems in cell mechanobiology, cancer biology, immune engineering, and stem cell-based regenerative medicine. He is the recipient of the American Heart Association Scientist Development Award, the Lab on a Chip Emerging Investigator Award, the New York University Whitehead Fellowship in Biomedical and Biological Sciences, the Goddard Junior Faculty Fellowship, the Baxter Young Investigator Award, the University of Michigan Richard F. & Eleanor A. Towner Prize for Outstanding PhD Research, and the ProQuest Distinguished Dissertation Award.

This article is part of the 2019 Young Innovators issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qian, W., Chen, W. Probing Single-Cell Mechanical Allostasis Using Ultrasound Tweezers. Cel. Mol. Bioeng. 12, 415–427 (2019). https://doi.org/10.1007/s12195-019-00578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00578-z