Abstract
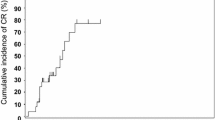
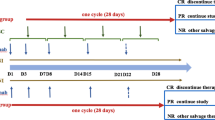
Steroid-refractory acute graft versus host disease (AGVHD) and chronic graft versus host disease (CGVHD) after allogeneic haematopoietic stem cell transplantation are major causes of morbidity and mortality. We undertook a phase I trial in patients with steroid-refractory AGVHD and CGVHD utilising bone marrow-derived mesenchymal stromal cells (MSC). Additionally, all refractory patients were treated with etanercept concomitantly. The primary end point was safety, and secondary end points were best response achieved and overall survival. A median of two infusions per patient were administered. The response rate overall for AGVHD was complete in seven, partial in four and no response in one patient. Of the seven patients who achieved a complete response, six are alive. The actuarial survival for the overall group of AGVHD was 55% at 30 months. Two patients with CGVHD achieved complete response with two partial responses and three with no response. The survival for those with AGVHD who achieved a complete response compared with those who did not was significant (p = 0.03). We identified no early or late safety issues in the nineteen patients. In view of the poor outlook for steroid-refractory AGVHD, further trials are warranted of MSC with steroid therapy, at the onset of AGVHD before steroid resistance.


Similar content being viewed by others
References
Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–26.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–94.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13(6):419–25.
Gotherstrom C. Immunomodulation by multipotent mesenchymal stromal cells. Transplantation. 2007;84(1 Suppl):S35–7.
Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8(2):110–23.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36.
Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(7):804–11.
Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–7.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–8.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11(12):945–56.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–6.
Kalwitz G, Andreas K, Endres M, Neumann K, Notter M, Ringe J, et al. Chemokine profile of human serum from whole blood: migratory effects of CXCL-10 and CXCL-11 on human mesenchymal stem cells. Connect Tissue Res. 2010;51(2):113–22.
Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10(8):771–4.
Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010;12(5):576–8.
Uberti JP, Ayash L, Ratanatharathorn V, Silver S, Reynolds C, Becker M, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11(9):680–7.
Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511–7.
Zhou H, Mei G, Chunjing B, et al. Efficacy of Bone marrow Derived Mesenchymal Stem Cells in the Treatment of Sclerodermatous Chronic Graft Versus Host Disease: Clinical Report. Biol Blood Marrow Transplant. 2010;16:403–12.
Zhang LS, Liu QF, Huang K, Zhang Y, Fan ZP, Huang SL. Mesenchymal stem cells for treatment of steroid-resistant chronic graft-versus-host disease. Zhonghua Nei Ke Za Zhi. 2009;48(7):542–6.
Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, et al. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45(12):1732–40.
Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal(TM)) in pediatric patients with severe refractory acute graft-versus-host disease in a Compassionate Use study. Biol Blood Marrow Transplant. 2010 (serial on the Internet).
Acknowledgments
This work was supported by The Ray and Bill Dobney Trust at the Royal Perth Hospital and by the Research and Infrastructure Support Services Company Ltd, funded by the Australian Federal Government National Consortium of Research Infrastructure Fund. We acknowledge the valuable contribution of the laboratory staff at CTTWA in manufacturing the MSC and the medical and nursing staff in caring for the patients.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Herrmann, R., Sturm, M., Shaw, K. et al. Mesenchymal stromal cell therapy for steroid-refractory acute and chronic graft versus host disease: a phase 1 study. Int J Hematol 95, 182–188 (2012). https://doi.org/10.1007/s12185-011-0989-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-011-0989-2