Abstract
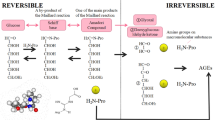
Individuals with diabetes have more atherosclerosis and cardiovascular events and a higher complication rate from cardiovascular events than nondiabetic individuals. Also, lesions from diabetic patients are more prone to rupture and exhibit more intravascular thrombosis than those from nondiabetic patients. Glucose modification of circulating proteins and matrix proteins mediates the activation of the inflammatory pathways critical in diabetes-associated atherosclerosis. Extracellular matrix proteins containing advanced glycation end products (AGEs) serve as ligands for scavenger receptors and the receptor for AGEs. Ligation of the receptor for AGEs activates inflammatory pathways and destabilizes atherosclerotic plaques in diabetic patients. Clinically, researchers have used concentrations of fructosamine and glycosylated hemoglobin to investigate the relationships among glycemia, glucose-modified proteins, and cardiovascular risk. They found that increased levels of fructosamine and hemoglobin A1c are associated with a significantly increased multivariable-adjusted risk of cardiovascular events.
Similar content being viewed by others
References and Recommended Reading
Sprafka JM, Burke GL, Folsom AR, et al.: Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care 1991, 14:537–543.
Miettinen H, Lehto S, Salomaa V, et al.: Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care 1998, 21:69–75.
Haffner SM, Lehto S, Ronnemaa T, et al.: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998, 339:229–234.
Rosenson, RS: The atherosclerotic milieu of diabetes. In Diabetes Management for Cardiologists. Edited by Brown AS. New York: Professional Publishing Group; In press.
Brizzi MF, Dentelli P, Pavan M, et al.: Diabetic LDL inhibits cell-cycle progression via STAT5B and p21(waf). J Clin Invest 2002, 109:111–119.
Morgan DO: Principles of CDK regulation. Nature 1995, 374:131–134.
van Brussel BL, Plokker HW, Voors AA, et al.: Multivariate risk factor analysis of clinical outcome 15 years after venous coronary artery bypass graft surgery. Eur Heart J 1995, 16:1200–1206.
Takazawa K, Hosoda Y, Yamamoto T, et al.: Coronary artery bypass grafting. Late result of actual 10-years follow-up in 376 patients. Jpn J Thorac Cardiovasc Surg 1999, 47:110–115.
Nishikawa T, Edelstein D, Du XL, et al.: Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404:787–790.
Basta G. Lazzerini G, Massaro M, et al.: Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation 2002, 105:816–822.
Masashi F, Hiroko O, Osamu T, et al.: Blockade of angiotensin II receptors reduces the expression of receptors for advanced glycation end products in human endothelial cells. Arterioscler Thromb Vasc Biol 2006, 26:e138–e142.
Cipollone F, Iezzi A, Fazia M, et al.: The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation 2003, 108:1070–1077.
Browner WS, Pressman AR, Lui LY, Cummings SR: Association between serum fructosamine and mortality in elderly women: the study of osteoporotic fractures. Am J Epidemiol 1999, 149:471–475.
Nathan DM, Singer DE, Hurxthal K, Goodson JD: The clinical information value of the glycosylated hemoglobin assay. N Engl J Med 1984, 310:341–346.
Goldstein DE, Little RR, Wiedmeyer HM, et al.: Glycated hemoglobin: methodologies and clinical applications. Clin Chem 1986, 32B:64–70.
Klein R: Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care 1995, 18:258–268.
Adler AI, Neil AW, Manley SE, et al.: Hyperglycemia and hyperinsulinemia at diagnosis of diabetes and their association with subsequent cardiovascular disease in the United Kingdom Prospective Diabetes Study (UKPDS 47). Am Heart J 1999, 138:S353–S359.
Stratton IM, Adler AI, Neil HA, et al.: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UPKDS 35): prospective observational study. BMJ 2000, 321:405–412.
Stevens RJ, Coleman RL, Adler AI, et al.: Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care 2004, 27:201–207.
Seltzer HS: A summary of criticisms of the findings and conclusions of the University Group Diabetic Program (UGDP). Diabetes 1972, 21:976–979.
Ohkubo Y, Kishikawa H, Araki E, et al.: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995, 28:103–117.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352:837–853.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352:854–865.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenson, R.S., Herman, W.H. Glycated proteins and cardiovascular disease in glucose intolerance and type 2 diabetes. Curr Cardio Risk Rep 2, 43–46 (2008). https://doi.org/10.1007/s12170-008-0009-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-008-0009-0