Abstract
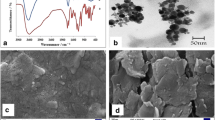
Monitoring of high intensity artificial sweeteners in the food industry for environmental and human health has become relevant in recent years. This work exploits the electrocatalytic properties of the laccase enzyme for sensing of a high intensity sweetener sucralose experimentally and supported by computational modeling. The fabrication of the laccase biosensor was achieved using laccase immobilized onto zinc oxide nanoparticles (ZnONPs) capped with p-amino thiophenol (ATP) and covalently attached to graphene oxide (GO) modified glassy carbon electrode (Lac/ZnONPs-ATP-GO/GCE). The developed biosensor exhibited an 8-fold enhancement of differential pulse voltammetry signals compared with the bare GCE at pH 5.0 in a 0.1 M phosphate buffer. The amplification of signals was due to a firm binding of laccase onto the surface of GO through high isoelectric point ZnONPs, exhibiting an enzymatic catalytic activity towards the oxidation of sucralose (SUC) at + 0.25 V (vs. Ag/AgCl). Under the optimized experimental conditions, the anodic peak current linearly increased with the sucralose concentrations ranging from 0.025–0.1 mM (R2 = 0.9984) and 0.25–1.0 mM (R2 = 0.9979) with a detection limit (S/N = 3) of 0.32 μM.
Furthermore, the proposed strategy was confirmed by assessing the interactions between the sucralose and the laccase using computational tools. First, the density functional theory (DFT) calculations of SUC revealed a HOMO–LUMO energy gap of − 0.2555 eV, suggesting a great tendency to act as an electron donor. Furthermore, adsorption and sucralose-laccase docking studies were carried out to better understand the redox mechanisms. These results revealed that SUC forms hydrogen bonds with ILE 230 and GLN 228 and other amino acids of the hydrophobic channel of the binding sites, thereby facilitating the redox reaction for the detection of sucralose.






Similar content being viewed by others
References
Adhikari B-R, Govindhan M, Chen A (2015) Sensitive detection of acetaminophen with graphene-based electrochemical sensor. Electrochim Acta 162:198–204
Aghaie A, Khanmohammadi A, Hajian A, Schmid U, Bagheri H (2019) Nonenzymatic electrochemical determination of paraoxon ethyl in water and fruits by graphene-based NiFe bimetallic phosphosulfide nanocomposite as a superior sensing layer. Food Anal Method 12:1545–1555. https://doi.org/10.1007/s12161-019-01486-8
Arends IW, Li Y-X, Ausan R, Sheldon RA (2006) Comparison of TEMPO and its derivatives as mediators in laccase catalysed oxidation of alcohols. Tetrahedron 62:6659–6665
Bathinapatla A, Kanchi S, Singh P, Sabela MI, Bisetty K (2016) An ultrasensitive performance enhanced novel cytochrome c biosensor for the detection of rebaudioside A. Biosens Bioelectron 77:116–123
Binder H (2006) Thermodynamics of competitive surface adsorption on DNA microarrays. J Phys Condens Matter 18:S491–S523. https://doi.org/10.1088/0953-8984/18/18/s02
Brizuela AB, Raschi AB, Castillo MV, Leyton P, Romano E, Brandán SA (2013) Theoretical structural and vibrational properties of the artificial sweetener sucralose. Comput Theor Chem 1008:52–60. https://doi.org/10.1016/j.comptc.2012.12.017
Chen X, Chen B (2015) Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ Sci Technol 49:6181–6189
Fabbrini M, Galli C, Gentili P (2002) Radical or electron-transfer mechanism of oxidation with some laccase/mediator systems. J Mol Catal B Enzym 18:169–171
Ghazizadeh AJ, Afkhami A, Bagheri H (2018) Voltammetric determination of 4-nitrophenol using a glassy carbon electrode modified with a gold-ZnO-SiO2 nanostructure. Microchim Acta 185:296. https://doi.org/10.1007/s00604-018-2840-4
Gollavelli G, Ling Y-C (2014) Magnetic and fluorescent graphene for dual modal imaging and single light induced photothermal and photodynamic therapy of cancer cells. Biomaterials 35:4499–4507
Gupta VK, Sadeghi R, Karimi F (2013) A novel electrochemical sensor based on ZnO nanoparticle and ionic liquid binder for square wave voltammetric determination of droxidopa in pharmaceutical and urine samples. Sens Actuators B Chem 186:603–609
Hanko VP, Rohrer JS (2004) Determination of sucralose in splenda and a sugar-free beverage using high-performance anion-exchange chromatography with pulsed amperometric detection. J Agric Food Chem 52:4375–4379
Ibupoto Z, Jamal N, Khun K, Liu X, Willander M (2013) A potentiometric immunosensor based on silver nanoparticles decorated ZnO nanotubes, for the selective detection of d-dimer. Sens Actuators B Chem 182:104–111
Idris M, Srivastava S, Baggi T, Shulka S, Ganjoo A (2010) Rhodamine-sulphuric acid-a new visualization reagent for the determination of sucralose by HPTLC. J Chem 7:S559–S565
Jaušovec D, Vogrinčič R, Kokol V (2015) Introduction of aldehyde vs. carboxylic groups to cellulose nanofibers using laccase/TEMPO mediated oxidation. Carbohydr Polym 116:74–85
Jenner M, Smithson A (1989) Physicochemical properties of the sweetener sucralose. J Food Sci 54:1646–1649
Jo S, Kim T, Iyer VG, Im W (2008) CHARMM-GUI: a web-based graphical user interface for CHARMM. J Comput Chem 29:1859–1865. https://doi.org/10.1002/jcc.20945
Jo S et al (2014) Chapter Eight - CHARMM-GUI PDB manipulator for advanced modeling and simulations of proteins containing nonstandard residues. In: Karabencheva-Christova T (ed) Adv Protein Chem Struct Biol, vol 96. Academic Press, pp 235–265. https://doi.org/10.1016/bs.apcsb.2014.06.002
Johns P, Dowlati L (2003) Determination of acesulfame and sucralose in oral electrolyte maintenance solution by liquid chromatography. J AOAC Int 86:79–85
Kanchi S, Sabela MI, Singh P, Bisetty K (2017) Multivariate optimization of differential pulse polarographic–catalytic hydrogen wave technique for the determination of nickel(II) in real samples. Arab J Chem 10:S2260–S2272. https://doi.org/10.1016/j.arabjc.2013.07.061
Karimian N, Hashemi P, Khanmohammadi A, Afkhami A, Bagheri H (2020) The principles and recent applications of bioelectrocatalysis. J Anal Bioanal Chem Res 7:281–28\. https://doi.org/10.22036/abcr.2020.206676.1423
Knight I (1994) The development and applications of sucralose, a new high-intensity sweetener. Can J Physiol Pharmacol 72:435–439
Kumar A, Singh D, Kumar D, Kumar D (2018) Quantum mechanical study of nucleic acid interaction with carbon nanotubes in interior and at exterior positions. Adv Sci Lett 24:802–806. https://doi.org/10.1166/asl.2018.10847
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem Interf Electrochem 101:19–28
Lv N, Guo T, Liu B, Wang C, Singh V, Xu X, Li X, Chen D, Gref R, Zhang J (2017) Improvement in thermal stability of sucralose by γ-cyclodextrin metal-organic frameworks. Pharm Res 34:269–278. https://doi.org/10.1007/s11095-016-2059-1
Mahmoudi E, Fakhri H, Hajian A, Afkhami A, Bagheri H (2019) High-performance electrochemical enzyme sensor for organophosphate pesticide detection using modified metal-organic framework sensing platforms. Bioelectrochemistry 130:107348. https://doi.org/10.1016/j.bioelechem.2019.107348
Martins CA, Fernández PS, de Lima F, Troiani HE, Martins ME, Arenillas A, Maia G, Camara GA (2014) Remarkable electrochemical stability of one-step synthesized Pd nanoparticles supported on graphene and multi-walled carbon nanotubes. Nano Energy 9:142–151
Mead RN, Morgan JB, Avery GB Jr, Kieber RJ, Kirk AM, Skrabal SA, Willey JD (2009) Occurrence of the artificial sweetener sucralose in coastal and marine waters of the United States. Mar Chem 116:13–17
Motwani HV, Qiu S, Golding BT, Kylin H, Törnqvist M (2011) Cob (I) alamin reacts with sucralose to afford an alkylcobalamin: relevance to in vivo cobalamin and sucralose interaction. Food Chem Toxicol 49:750–757
Pham TA, Choi BC, Lim KT, Jeong YT (2011) A simple approach for immobilization of gold nanoparticles on graphene oxide sheets by covalent bonding. Appl Surf Sci 257:3350–3357
Roberts A, Renwick A, Sims J, Snodin D (2000) Sucralose metabolism and pharmacokinetics in man. Food Chem Toxicol 38:31–41
Sanghavi BJ, Kalambate PK, Karna SP, Srivastava AK (2014) Voltammetric determination of sumatriptan based on a graphene/gold nanoparticles/Nafion composite modified glassy carbon electrode. Talanta 120:1–9
Sarma H, Sarma K (2014) X-ray peak broadening analysis of ZnO nanoparticles derived by precipitation method. Int J Sci Res Pub 4:1–7
Sharma D, Sharma S, Kaith B, Rajput J, Kaur M (2011) Synthesis of ZnO nanoparticles using surfactant free in-air and microwave method. Appl Surf Sci 257:9661–9672
Sheikhi M, Shahab S, Khaleghian M, Kumar R (2018) Interaction between new anti-cancer drug syndros and CNT(6,6-6) nanotube for medical applications: geometry optimization, molecular structure, spectroscopic (NMR, UV/Vis, excited state), FMO, MEP and HOMO-LUMO investigation. Appl Surf Sci 434:504–513. https://doi.org/10.1016/j.apsusc.2017.10.154
Skálová T, Dohnálek J, Østergaard LH, Østergaard PR, Kolenko P, Dušková J, Štěpánková A, Hašek J (2009) The structure of the small laccase from Streptomyces coelicolor reveals a link between laccases and nitrite reductases. J Mol Biol 385:1165–1178. https://doi.org/10.1016/j.jmb.2008.11.024
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2606. https://doi.org/10.1021/cr950046o
Soltani-Shahrivar M, Karimian N, Fakhri H, Hajian A, Afkhami A, Bagheri H (2019) Design and application of a non-enzymatic sensor based on metal-organic frameworks for the simultaneous determination of carbofuran and carbaryl in fruits and vegetables. Electroanal 31:2455–2465. https://doi.org/10.1002/elan.201900363
Stroka J, Dossi N, Anklam E (2003) Determination of the artificial sweetener Sucralose® by capillary electrophoresis. Food Addit Contam 20:524–527
Wang K, Liu P, Ye Y, Li J, Zhao W, Huang X (2014) Fabrication of a novel laccase biosensor based on silica nanoparticles modified with phytic acid for sensitive detection of dopamine. Sens Actuators B Chem 197:292–299
Xu X, Lu P, Zhou Y, Zhao Z, Guo M (2009) Laccase immobilized on methylene blue modified mesoporous silica MCM-41/PVA. Mater Sci Eng C 29:2160–2164
Yan W, Wang N, Zhang P, Zhang J, Wu S, Zhu Y (2016) Simultaneous determination of sucralose and related compounds by high-performance liquid chromatography with evaporative light scattering detection. Food Chem 204:358–364
Yola ML, Atar N, Üstündağ Z, Solak AO (2013) A novel voltammetric sensor based on p-aminothiophenol functionalized graphene oxide/gold nanoparticles for determining quercetin in the presence of ascorbic acid. J Electroanal Chem 698:9–16
Zhang Z, Fu X, Li K, Liu R, Peng D, He L, Wang M, Zhang H, Zhou L (2016) One-step fabrication of electrochemical biosensor based on DNA-modified three-dimensional reduced graphene oxide and chitosan nanocomposite for highly sensitive detection of Hg (II). Sens Actuators B Chem 225:453–462
Zygler A, Wasik A, Kot-Wasik A, Namieśnik J (2012) The content of high-intensity sweeteners in different categories of foods available on the Polish market. Food Addit Contam 29:1391–1401
Acknowledgments
The authors would like to extend their gratitude to the National Research Foundation of South Africa for their financial assistance. KB acknowledges the Centre for High Performance Computing (CHPC), Cape Town, South Africa for all the computational resources.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 257 kb)
Rights and permissions
About this article
Cite this article
Bathinapatla, A., Kanchi, S., Sabela, M.I. et al. Experimental and Computational Studies of a Laccase Immobilized ZnONPs/GO-Based Electrochemical Enzymatic Biosensor for the Detection of Sucralose in Food Samples. Food Anal. Methods 13, 2014–2027 (2020). https://doi.org/10.1007/s12161-020-01824-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-020-01824-1