Abstract
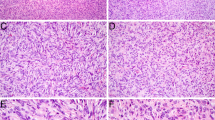
GLI1 fusions involving ACTB, MALAT1, PTCH1 and FOXO4 genes have been reported in a subset of malignant mesenchymal tumors with a characteristic nested epithelioid morphology and frequent S100 positivity. Typically, these multilobulated tumors consist of uniform epithelioid cells with bland nuclei and are organized into distinct nests and cords with conspicuously rich vasculature. We herein expand earlier findings by reporting a case of a 34-year-old female with an epithelioid mesenchymal tumor of the palate. The neoplastic cells stained positive for S100 protein and D2-40, whereas multiple other markers were negative. Genetic alterations were investigated by targeted RNA sequencing, and a PTCH1-GLI1 fusion was detected. Epithelioid mesenchymal tumors harboring a PTCH1-GLI1 fusion are vanishingly rare with only three cases reported so far. Due to the unique location in the mucosa of the soft palate adjacent to minor salivary glands, multilobulated growth, nested epithelioid morphology, focal clearing of the cytoplasm, and immunopositivity for S100 protein and D2-40, the differential diagnoses include primary salivary gland epithelial tumors, in particular myoepithelioma and myoepithelial carcinoma. Another differential diagnostic possibility is the ectomesenchymal chondromyxoid tumor. Useful diagnostic clues for tumors with a GLI1 rearrangement include a rich vascular network between the nests of neoplastic cells, tumor tissue bulging into vascular spaces, and absence of SOX10, GFAP and cytokeratin immunopositivity. Identifying areas with features of GLI1-rearranged tumors should trigger subsequent molecular confirmation. This is important for appropriate treatment measures as PTCH1-GLI1 positive mesenchymal epithelioid neoplasms have a propensity for locoregional lymph node and distant lung metastases.





Similar content being viewed by others
Data Availability
Data supporting the findings of this study are available within the article and its supplementary information files.
Code Availability
Not applicable.
References
Dahlén A, Fletcher CD, Mertens F, et al. Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12). Am J Pathol. 2004;164(5):1645–53.
Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, Dickson BC. A distinct malignant epithelioid neoplasm with GLI1 gene rearrangements, frequent S100 protein expression, and metastatic potential: expanding the spectrum of pathologic entities with ACTB/MALAT1/PTCH1-GLI1 fusions. Am J Surg Pathol. 2018;42(4):553–60.
Agaram NP, Zhang L, Sung YS, et al. GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod Pathol. 2019;32:1617–26.
Koh NWC, Seow WY, Lee YT, Lam JCM, Lian DWQ. Pericytoma with t(7;12): the first ovarian case reported and a review of the literature. Int J Gynecol Pathol. 2019;38(5):479–84.
Bridge JA, Sanders K, Huang D, et al. Pericytoma with t(7;12) and ACTB-GLI1 fusion arising in bone. Hum Pathol. 2012;43(9):1524–9.
Prall OWJ, McEvoy CRE, Byrne DJ, et al. A malignant neoplasm from the jejunum with a MALAT1-GLI1 fusion and 26-year survival history. Int J Surg Pathol. 2020;28(5):553–62.
Graham RP, Nair AA, Davila JI, et al. Gastroblastoma harbors a recurrent somatic MALAT1–GLI1 fusion gene. Mod Pathol. 2017;30(10):1443–52.
Spans L, Fletcher CDM, Antonescu CR, et al. Recurrent MALAT1–GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J Pathol. 2016;239(3):335–43.
Castro E, Cortes-Santiago N, Suarez Ferguson LM, Rao PH, Venkatramani R, López-Terrada D. Translocation t(7;12) as the sole chromosomal abnormality resulting in ACTB-GLI1 fusion in pediatric gastric pericytoma. Hum Pathol. 2016;53:137–41.
Kerr DA, Pinto A, Subhawong TK, et al. Pericytoma with t(7;12) and ACTB-GLI1 fusion: reevaluation of an unusual entity and its relationship to the spectrum of GLI1 fusion-related neoplasms. Am J Surg Pathol. 2019;43(12):1682–92.
Xu B, Chang K, Fople AL, et al. Head and neck mesenchymal neoplasms with GLI1 gene alterations: a pathologic entity with distinct histologic features and potential for distant metastasis. Am J Surg Pathol. 2020;44(6):729–36.
Ichikawa D, Yamashita K, Okuno Y, et al. Integrated diagnosis based on transcriptome analysis in suspected pediatric sarcomas. NPJ Genomic Med. 2021;6:49.
Dalin MG, Katabi N, Persson M, et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat Commun. 2017;8(1):1197.
Skálová A, Agaimy A, Vanecek T, et al. Molecular profiling of clear cell myoepithelial carcinoma of salivary glands with EWSR1 rearrangement identifies frequent PLAG1 gene fusions but no EWSR1 fusion transcripts. Am J Surg Pathol. 2021;45(1):1–13.
Pettus JR, Kerr DA, Stan RV, et al. Primary myxoid and epithelioid mesenchymal tumor of the kidney with a novel GLI1-FOXO4 fusion. Genes Chromosom Cancer. 2021;60(2):116–22.
Sari IN, Phi LTH, Jun N, Wijaya YT, Lee S, Kwon HY. Hedgehog signaling in cancer: a prospective therapeutic target for eradicating cancer stem cells. Cells. 2018;7(11):208.
Carpenter RL, Lo HW. Hedgehog pathway and GLI1 isoforms in human cancer. Discov Med. 2012;13(69):105–13.
Laco J, Mottl R, Höbling W, et al. Cyclin D1 expression in ectomesenchymal chondromyxoid tumor of the anterior tongue. Int J Surg Pathol. 2016;24(7):586–94.
Dickson BC, Antonescu CR, Argyris PP, et al. Ectomesenchymal chondromyxoid tumor: a neoplasm characterized by recurrent RREB1-MKL2 fusions. Am J Surg Pathol. 2018;42(10):1297–305.
Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo HW. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel). 2016;8(2):22.
Acknowledgements
This study was supported by study grant SVV 22639 from the Ministry of Education, Czech Republic (NK).
Funding
This study was supported by study grant SVV 22639 from the Ministerstvo školství, mládeže a tělovýchovy, Czech Republic (NK).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AS, JM and VH. The first draft of the manuscript was written by NK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
The study was approved by the institutional review board of the Faculty of Medicine in Pilsen, Charles University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed Consent
No patient consent was required for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klubíčková, N., Kinkor, Z., Michal, M. et al. Epithelioid Soft Tissue Neoplasm of the Soft Palate with a PTCH1-GLI1 Fusion: A Case Report and Review of the Literature. Head and Neck Pathol 16, 621–630 (2022). https://doi.org/10.1007/s12105-021-01388-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01388-4