Abstract
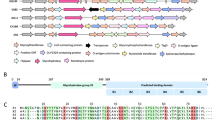
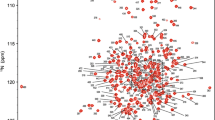
FtsX is an integral membrane protein from Streptococcus pneumoniae (pneumococcus) that harbors an extracellular loop 1 domain (\({\text{FtsX}}^{\text{ECL1}}_{Spn}\)) that interacts with PcsB, an peptidoglycan hydrolase that is essential for cell growth and division. Here, we report nearly complete backbone and side chain resonance assignments and a secondary structural analysis of \({\text{FtsX}}^{\text{ECL1}}_{Spn}\) (residues 47–168 of FtsX) as first steps toward structure determination of \({\text{FtsX}}^{\text{ECL1}}_{Spn}\).


Similar content being viewed by others
References
Bartual SG, Straume D, Stamsas GA, Munoz IG, Alfonso C, Martinez-Ripoll M, Havarstein LS, Hermoso JA (2014) Structural basis of PcsB-mediated cell separation in Streptococcus pneumoniae. Nat Commun 5:3842
Bax A, Clore GM, Gronenborn AM (1990) 1H-1H correlation via isotropic mixing of 13C magnetization, a new three-dimensional approach for assigning 1H and 13C spectra of 13C-enriched proteins. J Magn Reson 88(2):425–431
Delaglio F, Grzesiek S, Vuister G, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6(3):277–293
Grzesiek S, Anglister J, Bax A (1993) Correlation of backbone amide and aliphatic side-chain resonances in 13C/15N-enriched proteins by isotropic mixing of 13C magnetization. J Magn Reson 101(1):114–119
Mavrici D, Marakalala MJ, Holton JM, Prigozhin DM, Gee CL, Zhang YJJ, Rubin EJ, Alber T (2014) Mycobacterium tuberculosis FtsX extracellular domain activates the peptidoglycan hydrolase, RipC. Proc Natl Acad Sci USA 111(22):8037–8042
Montelione GT, Lyons BA, Emerson SD, Tashiro M (1992) An efficient triple resonance experiment using carbon-13 isotropic mixing for determining sequence-specific resonance assignments of isotopically-enriched proteins. J Am Chem Soc 114(27):10974–10975
Sham LT, Barendt SM, Kopecky KE, Winkler ME (2011) Essential PcsB putative peptidoglycan hydrolase interacts with the essential FtsX(Spn) cell division protein in Streptococcus pneumoniae D39. Proc Natl Acad Sci USA 108(45):E1061–E1069
Sham LT, Jensen KR, Bruce KE, Winkler ME (2013) Involvement of FtsE ATPase and FtsX extracellular loops 1 and 2 in FtsEX-PcsB complex function in cell division of Streptococcus pneumoniae D39. MBio 4(4):e00431-13. doi:10.1128/mBio.00431-13
Sheffield P, Garrard S, Derewenda Z (1999) Overcoming expression and purification problems of RhoGDI using a family of “parallel” expression vectors. Protein Expr Purific 15(1):34–39
Shen Y, Delaglio F, Cornilescu G, Bax A (2009) TALOS plus : a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR 44(4):213–223
Acknowledgments
This work was supported by NIH Grants GM042569 (to D. P. G.), GM114315 (to M. E. W.) and a predoctoral Quantitative and Chemical Biology (QCB) institutional training grant fellowship supported by T32 GM109825 (to B.R.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, Y., Bruce, K.E., Rued, B. et al. 1H, 13C, 15N resonance assignments of the extracellular loop 1 domain (ECL1) of Streptococcus pneumoniae D39 FtsX, an essential cell division protein. Biomol NMR Assign 10, 89–92 (2016). https://doi.org/10.1007/s12104-015-9644-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12104-015-9644-9