Abstract
Purpose
Both nintedanib/docetaxel and anti‐PD‐1/PD‐L1 immunotherapies have demonstrated efficacy as second‐line treatment of patients with advanced lung adenocarcinoma. This is the first report on the efficacy of the nintedanib/docetaxel combination following first‐line platinum‐based chemotherapy and subsequent immunotherapy in a real-world setting.
Methods/patients
From May 2014 to December 2015, 390 patients in 108 Spanish centres enrolled in the nintedanib named patient use program. Inclusion criteria were advanced lung adenocarcinoma with progressive disease following at least one line of platinum‐based doublet chemotherapy. The objective was to evaluate the efficacy of the nintedanib/docetaxel combination in patients who also received immunotherapy.
Results
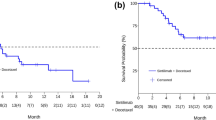
Eleven patients met the inclusion criteria; with a median age of 67 years. PD‐L1 expression was positive in six patients. Median progression‐free survival (PFS) of first‐line platinum‐based chemotherapy was 3.3 months (95% CI 1.9–4.6). Second‐line immunotherapy was pembrolizumab (36.5%), atezolizumab (36.5%) or nivolumab (27%). Median PFS of second‐line immunotherapy was 2.3 months (95% CI 0–6.1). The overall response rate (ORR) to second‐line immunotherapy was 18% with a disease‐control rate (DCR) of 45%. Median PFS of nintedanib/docetaxel was 3.2 months (95% CI 1.9–4.5). Best response was partial response in four patients (36%), stable disease in five patients (46%), and progressive disease in two patients (18%), for an ORR of 36% and a DCR of 82%.
Conclusion
Our experience suggests an encouraging efficacy of nintedanib/docetaxel in patients with adenocarcinoma NSCLC pretreated with platinum‐based doublet chemotherapy and immunotherapy, reinforcing the importance of an optimal therapeutic sequence for managing advanced lung adenocarcinoma.



Similar content being viewed by others
References
Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–55. https://doi.org/10.1016/S1470-2045(13)70586-2.
Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–73. https://doi.org/10.1016/S0140-6736(14)60845-X.
Gottfried M, Bennouna J, Bondarenko I, Douillard JY, Heigener DF, Krzakowski M, et al. Efficacy and safety of nintedanib plus docetaxel in patients with advanced lung adenocarcinoma: complementary and exploratory analyses of the phase III LUME-Lung 1 study. Target Oncol. 2017;12(4):475–85. https://doi.org/10.1007/s11523-017-0517-2.
Reck M, Garassino MC, Imbimbo M, Shepherd FA, Socinski MA, Shih JY, et al. Antiangiogenic therapy for patients with aggressive or refractory advanced non-small cell lung cancer in the second-line setting. Lung Cancer. 2018;120:62–9. https://doi.org/10.1016/j.lungcan.2018.03.025.
Reck M, Mellemgaard A, Novello S, Postmus PE, Gaschler-Markefski B, Kaiser R, et al. Change in non-small-cell lung cancer tumor size in patients treated with nintedanib plus docetaxel: analyses from the Phase III LUME-Lung 1 study. Onco Targets Ther. 2018;11:4573–82. https://doi.org/10.2147/OTT.S170722.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643.
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924–33. https://doi.org/10.1200/JCO.2017.74.3062.
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–50. https://doi.org/10.1016/S0140-6736(15)01281-7.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. https://doi.org/10.1056/NEJMoa1501824.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–46. https://doi.org/10.1016/S0140-6736(16)00587-0.
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–65. https://doi.org/10.1016/S0140-6736(16)32517-X.
Commmitee for Medicinal Products for Human Use (CHMP). European Medicines Agency Assessment report of Opdivo. EMA/246304/2016. 2016.
Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–26. https://doi.org/10.1056/NEJMoa1613493.
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–104. https://doi.org/10.1056/NEJMoa1801946.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–33. https://doi.org/10.1056/NEJMoa1606774.
National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology. Non-small cell lung cancer. Version 1.2019—October 19, 2018.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv192–237. https://doi.org/10.1093/annonc/mdy275.
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. https://doi.org/10.1056/NEJMoa1801005.
Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–508. https://doi.org/10.1016/S1470-2045(16)30498-3.
Lopes G, Wu Y-L, Kudaba I, Kowalski D, Cho BC, Castro G et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(18_suppl):abstr LBA4. https://doi.org/10.1200/jco.2018.36.18_suppl.lba4.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–301. https://doi.org/10.1056/NEJMoa1716948.
Leger PD, Rothschild S, Castellanos E, Pillai RN, York SJ, Horn L. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non-small cell lung cancer. J Clin Oncol. 2017;35(15_suppl):abstr 9084. https://doi.org/10.1200/jco.2017.35.15_suppl.9084.
Schvartsman G, Peng SA, Bis G, Lee JJ, Benveniste MFK, Zhang J, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–5. https://doi.org/10.1016/j.lungcan.2017.07.034.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13(1):106–11. https://doi.org/10.1016/j.jtho.2017.10.011.
Grigg C, Reuland BD, Sacher AG, Yeh R, Rizvi NA, Shu CA. Clinical outcomes of patients with non-small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. J Clin Oncol. 2017;35(15_suppl):abstr 9082. https://doi.org/10.1200/jco.2017.35.15_suppl.9082.
Brahmer JR, Rodriguez-Abreu D, Robinson AG, Hui R, Csõszi T, Fülöp A et al. Progression after the next line of therapy (PFS2) and updated OS among patients (pts) with advanced NSCLC and PD-L1 tumor proportion score (TPS) ≥ 50% enrolled in KEYNOTE-024. J Clin Oncol. 2017;35(15_suppl):abstr 9000. https://doi.org/10.1200/jco.2017.35.15_suppl.9000.
Costantini A, Corny J, Fallet V, Renet S, Friard S, Chouaid C, et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res. 2018. https://doi.org/10.1183/23120541.00120-2017.
Gettinger SN, Wurtz A, Goldberg SB, Rimm D, Schalper K, Kaech S, et al. Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non-small cell lung cancer. J Thorac Oncol. 2018;13(6):831–9. https://doi.org/10.1016/j.jtho.2018.03.008.
Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. https://doi.org/10.1038/cdd.2013.67.
Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2(10):e27025. https://doi.org/10.4161/onci.27025.
Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology. 2017;6(7):e1331807. https://doi.org/10.1080/2162402X.2017.1331807.
Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. https://doi.org/10.1200/JCO.2009.26.7609.
Hilberg F, Reschke M, Hofmann M, Kraut N. P2.01-045 Nintedanib improves anti-tumor efficacy in combination with anti PD-1 in syngeneic tumor models sensitive and refractory to IO inhibition. J Thorac Oncol. 2017;12(1):S813. abstr P2.01-045. https://doi.org/10.1016/j.jtho.2016.11.1097.
Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol. 2015;33:55–63. https://doi.org/10.1016/j.coi.2015.01.011.
Garde-Noguera J, Martin-Martorell P, De Julian M, Perez-Altozano J, Salvador-Coloma C, Garcia-Sanchez J, et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non-small-cell lung cancer patients. Clin Transl Oncol. 2018;20(8):1072–9. https://doi.org/10.1007/s12094-017-1829-5.
Peters S, Gettinger S, Johnson ML, Janne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–9. https://doi.org/10.1200/JCO.2016.71.9476.
Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19(4):521–36. https://doi.org/10.1016/S1470-2045(18)30144-X.
Funding
The nintedanib NPU was funded by Boehringer Ingelheim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have not conflict of interest.
Ethical approval
This study was approved by the Ethics Committees of the participating hospitals and the procedures were performed in accordance with ethical standards of the Institutions′ Research Committees and with the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All patients provided written informed prior to their inclusion in the study. Data that might disclose the identity of the patient has been omitted and the confidentiality of the patient data has been maintained throughout the study and the analyses.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Corral, J., Majem, M., Rodríguez-Abreu, D. et al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin Transl Oncol 21, 1270–1279 (2019). https://doi.org/10.1007/s12094-019-02053-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02053-7