Abstract
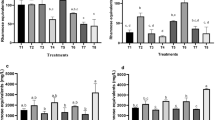
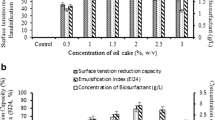
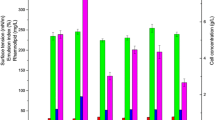
Environmental awareness has led to a serious consideration for biological surfactants and hence non-edible vegetable oils may serve as a substitute carbon source for bio-surfactant production (rhamnolipid) which might be an alternative to complex synthetic surfactants. There are reports of rhamnolipid production from plant based oil giving higher production than that of glucose because of their hydrophobicity and high carbon content. Therefore the contribution of non-edible oil such as Mesua ferrea seed oil could serve as a good carbon source for rhamnolipid production. Moreover the use of rhamnolipid production from non-edible plant based seed oil has not been reported elsewhere. The present work focus on the optimal production of rhamnolipid by considering both micro and macro nutrients and culture conditions using response surface methodology. The study observes that micronutrients play a significant role in rhamnolipid production from Pseudomonas aeruginosa (MTCC 7815). The investigation results with the statistically optimize parameters able to produce a higher rhamnolipid production and this methodology could be used to optimize the nutrients requirements and culture conditions. The present findings would assist in bioremediation of crude oil contaminated ecosystems.


Similar content being viewed by others
References
Nitschke M, Costa SG, Haddad R, Gonçalves LA, Eberlin MN, Contiero J (2005) Oil wastes as unconventional substrates for rhamnolipid biosurfactant production by Pseudomonas aeruginosa LBI. Biotechnol Prog 21(5):1562–1566
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53(5):495–508
Wei YH, Cheng CL, Chien CC, Wan HM (2008) Enhanced di-rhamnolipid production with an indigenous isolate Pseudomonas aeruginosa J16. Process Biochem 43:769–774
Rahman KS, Rahman TJ, Kourkoutas Y, Petsas I, Marchant R, Banat IM (2003) Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol 90(2):159–168
Kitamoto D, Isoda H, Nakahara T (2002) Functions and potential applications of glycolipid biosurfactants–from energy-saving materials to gene delivery carriers. J Biosci Bioeng 94(3):187–201
Maier RM, Soberón-Chávez G (2000) Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biotechnol 54(5):625–633
del Pozo-Rodríguez A, Delgado D, Solinís MA, Pedraz JL, Echevarría E, Rodríguez JM, Gascón AR (2010) Solid lipid nanoparticles as potential tools for gene therapy: in vivo protein expression after intravenous administration. Int J Pharm 385:157–162
Cevc G, Vierl U (2010) Nanotechnology and transdermal rout: a state of the art review and critical appraisal. J Control Release 141:277–299
Rodrigues L, Moldes A, Teixeira J, Oliveira R (2006) Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochem Eng J 28(2):109–116
Makkar RS, Cameotra SS (1999) Biosurfactant production by microorganisms on unconventional carbon sources—a review. J Surf Det 2:237–241
Mercade ME, Manresa MA, Robert M, Espuny MJ, Andres C, Guinea J (1993) Olive oil mill effluent (OOME). New substrate for biosurfactant production. Bioresour Technol 43:1–6
Oliveira FJS, Vazquez L, de Campos NP, de França FP (2009) Production of rhamnolipids by a Pseudomonas alcaligenes strain. Process Biochem 44:383–389
Manresa MA, Bastida J, Mercade ME, Robert M, de Andres C, Espung MJ et al (1991) Kinetic studies on surfactant production by Pseudomonas aeruginosa 4471. J Ind Microbiol 8:133–136
Benincasa M, Contiero J, Manresa MA, Moraes IO (2002) Rhamnolipid production by Pseudomonas aeruginosa LBI growing on soapstock as the sole carbon source. J Food Eng 54:283–288
Wu JY, Yeh KL, Lu WB, Lin CL, Chang JS (2008) Rhamnolipid production with indigenous Pseudomonas aeruginosa EM1 isolated from oil-contaminated site. Bioresour Technol 99(5):1157–1164
Rahman KS, Rahman TJ, McClean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactants production by strains of Pseudomonas aeruginosa using low cost raw materials. Biotechnol Prog 18:277–281
Sim L, Ward OP, Li ZY (1997) Production and characterisation of a biosurfactant isolated from Pseudomonas aeruginosa UW-1. J Ind Microbiol Biotechnol 19:232–238
Mata-Sandoval JC, Karns J, Torrents A (1999) High-performance liquid chromatography method for the characterization of rhamnolipid mixtures produced by Pseudomonas aeruginosa UG2 on corn oil. J Chromatogr A 864:211–220
Ko-Sin N, Wei-Yang O, Lay-Koon G, Rajaiah S, Kumar S (2010) Evaluation of jatropha oil to produce poly(3-hydroxybutyrate) by Cupriavidus necator H16. Polym Degrad Stab 95:1365–1369
Kostermans AJGH (1980) Clusiaceae (Guttiferae). In: Dassanayaka MD, Fosberg FR (eds) A revised handbook to the flora of Ceylon. Amerind, New Delhi, pp 107–110
Sayeed MA, Ali MA, Sohel FI, Khan AM, Yeasmin MS (2004) Physico-chemical characteristics of Mesua ferrea seed oil and nutritional composition of its seed and leaves. Bull Chem Soc Ethiop 18(2):157–166
Rao KJ, Kim CH, Rhee SK (2000) Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Process Biochem 35:639–647
Kunamneni A, Singh S (2005) Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem Eng J 27:179–190
Saeed HM, Abdel-Fattah YR, Gohar YM, Elbaz MA (2005) Improved production of Pseudomonas aeruginosa uricase by optimization of process parameters through statistical experimental designs. Process Biochem 40:1707–1714
Song L, Qin JG, Su S, Xu J, Clarke S et al (2012) Micronutrient requirements for growth and hydrocarbon production in the oil producing green alga Botryococcus braunii (Chlorophyta). PLoS ONE 7(7):e41459
Meyer-Hoffert U, Zimmermann A, Czapp M, Bartels J, Koblyakova Y et al (2011) Flagellin delivery by Pseudomonas aeruginosa rhamnolipids induces the antimicrobial protein psoriasin in human skin. PLoS ONE 6(1):e16433
Kamatkar NG, Shrout JD (2011) Surface hardness impairment of quorum sensing and swarming for Pseudomonas aeruginosa. PLoS ONE 6(6):e20888
Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK et al (2010) Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS ONE 5(4):e10115
Macé C, Seyer D, Chemani C, Cosette P, Di-Martino P et al (2008) Identification of biofilm-associated cluster (bac) in Pseudomonas aeruginosa involved in biofilm formation and virulence. PLoS ONE 3(12):e3897
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Bharali P, Konwar BK (2011) Production and physico-chemical characterization of a biosurfactant produced by Pseudomonas aeruginosa OBP1 isolated from petroleum sludge. Appl Biochem Biotechnol 164(8):1444–1460
Strobel RJ, Sullivan GR (1999) Experimental design for improvement of fermentations. In: Demain AL, Davies GE (eds) Manual of industrial microbiology and biotechnology. ASM, Washington, DC, pp 80–93
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475
Minitab 15 Statistical Software, Minitab Inc., PA, USA
Maier RM, Soberon-Chavez G (2000) Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biotechnol 54:625–633
Sandoval JCM, Karns J, Torrents A (2001) Effect of nutritional and environmental conditions on the production and composition of rhamnolipids by Pseudomonas aeruginosa UG2. Microbiol Res 155:249–256
Costa SGVAO, Nitschke M, Haddad R, Eberlin MN, Contiero J (2006) Production of Pseudomonas aeruginosa LBI rhamnolipids following growth on Brazilian native oils. Proc Biochem 41:483–488
Rashedi H, Mazaheri AM, Jamshidi E, Bonakdarpour B (2006) Optimization of the production of biosurfactant by Psuedomonas aeruginosa HR Isolated from an Iranian southern oil well. Iran J Chem Chem Eng 25:25–30
Bordoloi NK, Konwar BK (2008) Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloids Surf B 63:73–82
Xia WJ, Luo ZB, Dong HP, Yu L, Cui QF, Bi YQ (2012) Synthesis, characterization, and oil recovery application of biosurfactant produced by indigenous pseudomonas aeruginosa WJ-1 using waste vegetable oils. Appl Biochem Biotechnol 166(5):1148–1166
El-Sersy NA, Ibrahim HAH, Abou-Elela GM (2010) Response surface methodology as a tool for optimizing the production of antimicrobial agents from Bacillus licheniformis SN2. Curr Res Bacteriol 3:1–14
Banik RM, Pandey SK (2009) Selection of metal salts for alkaline phosphatase production using response surface methodology. Bioprocess Food Ind 42:470–475
Acknowledgments
The authors would like to thank Yasser R. Abdel-Fattah, Bioprocess Development Department, Genetic Engineering and Biotechnology Research Institute, Alexandria, Egypt for his valuable suggestions in carrying out the statistical analysis part.
Conflict of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, S.P., Bharali, P. & Konwar, B.K. Optimization of Nutrient Requirements and Culture Conditions for the Production of Rhamnolipid from Pseudomonas aeruginosa (MTCC 7815) using Mesua ferrea Seed Oil. Indian J Microbiol 53, 467–476 (2013). https://doi.org/10.1007/s12088-013-0403-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-013-0403-2