Abstract
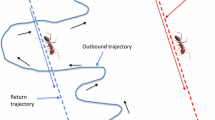
Returning to the point of departure after exploring the environment is a key capability for most animals. In the absence of landmarks, this task will be solved by integrating direction and distance traveled over time. This is referred to as path integration or dead reckoning. An important question is how the nervous systems of navigating animals such as the 1 mm3 brain of ants can integrate local information in order to make global decision. In this article we propose a neurobiologically plausible system of storing and retrieving direction and distance information. The path memory of our model builds on the well established concept of population codes, moreover our system does not rely on trigonometric functions or other complex non-linear operations such as multiplication, but only uses biologically plausible operations such as integration and thresholding. We test our model in two paradigms; in the first paradigm the system receives input from a simulated compass, in the second paradigm, the model is tested against behavioral data recorded from 17 ants. We were able to show that our path memory system was able to reliably encode and compute the angle of the vector pointing to the start location, and that the system stores the total length of the trajectory in a dependable way. From the structure and behavior of our model, we derive testable predictions both at the level of observable behavior as well as on the anatomy and physiology of its underlying neuronal substrate.















Similar content being viewed by others
References
Angstadt J (1999) Persistent inward currents in cultured Retzius cells of the medicinal leech. J Comp Physiol [A] 184:49–61
Bernardet U (2007) iqr–large-scale neural systems simulator http://iqr.sourceforge.net
Bernardet U, Blanchard M, Verschure P (2002) Iqr: a distributed system for real-time real-world neuronal simulation. Neurocomputing 44-46:1043–1048
Darwin C (1873) Origin of certain instincts. Nature 7:417–418
Egorov A, Hamam B, Fransén E, Hasselmo M, Alonso A (2002) Graded persistent activity in entorhinal cortex neurons. Nature 420:173–178
Elias S, Grossberg S (1975) Pattern formation, contrast control and oscillations in the short term memory of shunting on-center off-surround networks. Biol Cybern (20):69–98
Esch H, Burns J (1996) Distance estimation by foraging honeybees. J Exp Biol 199:155–162
Etienne A, Jeffery K (2004) Path integration in mammals. Hippocampus 14:180–192
Feldman J, Ballard D (1982) Connectionist models and their properties. Cognit Sci 6:205–254
Fukushima K (1980) Neocognitron: a self organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol Cybern 36(4):193–202. 0340-1200 (Print) Journal Article
Gabbiani F, Krapp H, Koch C, Laurent G (2002) Multiplicative computation in a visual neuron sensitive to looming. Nature 420:320–324
Georgopoulos A (1994) New concepts in generation of movement. Neuron 13:257–268
Georgopoulos A, Schwartz A, Kettner R (1986) Neuronal population coding of movement direction. Science 233:1416–1419
Hartmann G, Wehner R (1995) The ant’s path integration system: a neural architecture. Biol Cybern 73:483–497
Hertz J, Krogh A, Palmer R (1991) Introduction to the theory of neural computation. Addison-Wesley, Redwood City
Homberg U, Würden S (1997) Movement-sensitive, polarization-sensitive, and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol 386:329–346
Hrncir M, Jarau S, Zucchi R, Barth F (2003) A stingless bee (Melipona seminigra) uses optic flow to estimate flight distances. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189:761–768
Jacobs G, Theunissen F (1996) Functional organization of a neural map in the cricket cercal sensory system. J Neurosci 16:769–784
Knierim J, Kudrimoti H, McNaughton B (1995) Place cells, head direction cells, and the learning of landmark stability. J Neurosci 15:1648–1659
Labhart T (1988) Polarization-opponent interneurones in the insect visual system. Nature 331:435–437
Labhart T, Meyer EP, Schenker L (1992) Specialized ommatidia for polarization vision in the compound eye of cockchafers, Melolontha melolontha (coleoptera, scarabaeidae). Cell Tissue Res 268 (3), 419–429
Lambrinos D (2003) Navigation in desert ants: the robotic solution. Robotica 21:407–426
Lambrinos D, Möller R, Labhart T, Pfeifer R, Wehner R (2000) A mobile robot employing insect strategies for navigation. Rob Auton Syst 30:39–64
Lewis J, Kristan W (1998) A neuronal network for computing population vectors in the leech. Nature 391:76–79
Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L (2006) Distinct memory traces for two visual features in the Drosophila brain. Nature 439:551–556
Loesel R, Homberg U (2001) Anatomy and physiology of neurons with processes in the accessory medulla of the cockroach Leucophaea maderae. J Comp Neurol 439:193–207
Major G, Tank D (2004) Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol 14:675–684
Marr D, Poggio T (1979) A computational theory of human stereo vision. Proc R Soc Lond B Biol Sci 204 (1156):301–328. 0080-4649 (Print) Journal Article
Menzel R, Greggers U, Smith A, Berger S, Brandt R, Brunke S, Bundrock G, Hülse S, Plümpe T, Schaupp F, Schüttler, E., Stach S, Stindt J, Stollhoff N, Watzl S (2005) Honey bees navigate according to a map-like spatial memory. Proc Natl Acad Sci U S A 102:3040–3045
Mercer A, Kloppenburg P, Hildebrand J (2005) Plateau potentials in developing antennal-lobe neurons of the moth, Manduca sexta. J Neurophysiol 93:1949–1958
Mittelstaedt ML, Mittelstaedt H (1980) Homing by path integration in a mammal. Naturwissenschaften 67:566–567
Möller R (2000) Insect visual homing strategies in a robot with analog processing. Biol Cybern 83:231–243
Mudra R, Douglas R (2003) Self-correction mechanism for path integration in a modular navigation system on the basis of an egocentric spatial map. Neural Netw 16:1373–1388
Pascual A, Préat T (2001) Localization of long-term memory within the Drosophila mushroom body. Science 294:1115–1117
Ramirez J, Pearson K (1991) Octopamine induces bursting and plateau potentials in insect neurones. Brain Res 549:332–337
Reinhard J, Srinivasan M, Zhang S (2006) Complex memories in honeybees: can there be more than two? J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192:409–416
Reppert S, Zhu H, White R (2004) Polarized light helps monarch butterflies navigate. Curr Biol 14:155–158
Ronacher B, Gallizzi K, Wohlgemuth S, Wehner R (2000) Lateral optic flow does not influence distance estimation in the desert ant Cataglyphis fortis. J Exp Biol 203:1113–1121
Rossel S, Wehner R (1986) Polarized vision in bees. Nature 323:128–131
Salinas E, Abbott L (1994) Vector reconstruction from firing rates. J Comput Neurosci 1:89–107
Si A, Srinivasan M, Zhang S (2003) Honeybee navigation: properties of the visually driven “odometer”. J Exp Biol 206:1265–1273
Sommer S, Wehner R (2004) The ant’s estimation of distance travelled: experiments with desert ants, Cataglyphis fortis. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190:1–6
Spero D (2004) A review of outdoor robotics research. Tech. Rep. MECSE-17-2004, Department of Electrical and Computer Systems Engineering, Monash University, Melbourne, Australia. URL:http://www.citeseer.ist.psu.edu/spero04review.html
Srinivasan M, Zhang S, Altwein M, Tautz J (2000) Honeybee navigation: nature and calibration of the "odometer". Science 287: 851–853
Stalleicken J, Mukhida M, Labhart T, Wehner R, Frost B, Mouritsen H (2005) Do monarch butterflies use polarized skylight for migratory orientation? J Exp Biol 208:2399–2408
Taube J (2007) The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci 30:181–207
Tautz J, Zhang S, Spaethe J, Brockmann A, Si A, Srinivasan M (2004) Honeybee odometry: performance in varying natural terrain. PLoS Biol 2(7):E 211
Verschure P, Voegtlin T, Douglas R (2003) Environmentally mediated synergy between perception and behaviour in mobile robots. Nature 425:620–624
Vickerstaff R, Di Paolo E (2005) Evolving neural models of path integration. J Exp Biol 208:3349–3366
von Frisch K (1993) The dance language and orientation of bees. Harvard University Press, Cambridge
von Philipsborn A, Labhart T (1990) A behavioural study of polarization vision in the fly, Musca domestica. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 167(6):737–743
Wallace D, Hines D, Pellis S, Whishaw I (2002) Vestibular information is required for dead reckoning in the rat. J Neurosci 22:10009–10017
Wehner R (1997) Orientation and communication in arthropodes, the ant’s celestial compass system: spectral and polarization channels. Birkhäuser, Basel pp 145–185
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189:579–588
Wehner R, Srinivasan M (2003) The Neurobiology of spatial behaviour, path integration in insects. Oxford University Press, Oxford, pp 9–30
Wittlinger M, Wehner R, Wolf H (2006) The ant odometer: stepping on stilts and stumps. Science 312:1965–1967
Wittmann T, Schwegler H (1995) Path integration - a network model. Biol Cybern 73(6):569–575. doi:10.1007/s004220050212
Wohlgemuth S, Ronacher B, Wehner R (2001) Ant odometry in the third dimension. Nature 411:795–798
Yu A, Giese M, Poggio T (2002) Biophysically plusible implementations of the maximum operation. Neural Comput 14: 2857–2888
Yuille A, Grzywacz N (1988) A computational theory for the perception of coherent visual motion. Nature 333(6168):71–74. 0028-0836 (Print) Journal Article
Zars T, Fischer M, Schulz R, Heisenberg M (2000a) Localization of a short-term memory in Drosophila. Science 288:672–675
Zars T, Wolf R, Davis R, Heisenberg M (2000b) Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: in search of the engram. Learn Mem 7:18–31
Zhang S, Bock F, Si A, Tautz J, Srinivasan M (2005) Visual working memory in decision making by honey bees. Proc Natl Acad Sci USA 102:5250–5255
Acknowledgments
The authors thank Dr Markus Knaden for providing them with the behavioral data of the ants. This project was supported through the Synthetic Forager (FP7-IST 217148) and Neurochem (FP7-IST 216916).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bernardet, U., Bermúdez i Badia, S. & Verschure, P.F. A model for the neuronal substrate of dead reckoning and memory in arthropods: a comparative computational and behavioral study. Theory Biosci. 127, 163–175 (2008). https://doi.org/10.1007/s12064-008-0038-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12064-008-0038-8