Abstract
Transient receptor potential melastatin-2 (TRPM2) channels are cation channels activated by oxidative stress and ADP-ribose (ADPR). Role of TRPM2 channels has been postulated in several neurological disorders, but, it has not been explored in animal models of Parkinson’s disease (PD). Thus, the role of TRPM2 and its associated poly (ADPR) polymerase (PARP) signaling pathways were investigated in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD rat model using TRPM2 inhibitor, 2-aminoethyl diphenyl borinate (2-APB), and PARP inhibitor, N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-(N,N-dimethylamino) acetamide hydrochloride (PJ-34). PD was induced by using a bilateral intranigral administration of MPTP in rats, and different parameters were evaluated. An increase in oxidative stress was observed, leading to locomotor and cognitive deficits in the PD rats. PD rats also showed an increased TRPM2 expression in the striatum and mid-brain accompanied by reduced expression of tyrosine hydroxylase (TH) in comparison to sham animals. Intraperitoneal administration of 2-APB and PJ-34 led to an improvement in the locomotor and cognitive deficits in comparison to MPTP-induced PD rats. These improvements were accompanied by a reduction in the levels of oxidative stress and an increase in TH levels in the striatum and mid-brain. In addition, these pharmacological interventions also led to a decrease in the expression of TRPM2 in PD in the striatum and mid-brain. Our results provide a rationale for the development of potent pharmacological agents targeting the TRPM2-PARP pathway to provide therapeutic benefits for the treatment of neurological diseases like PD.






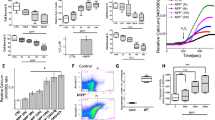
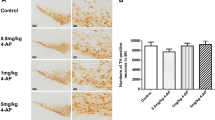
taken from different gels and ACTIN, TH and TRPM2 exposures with their antibodies are different. Quantification for blots is shown in d TH in striatum, e TRPM2 in striatum, and f TRPM2 in mid-brain. Results were expressed as mean ± SEM. *p < 0.05 vs. sham animals; #p < 0.05, ##p < 0.01 vs. MPTP-induced PD animals (one-way ANOVA)

Similar content being viewed by others
Data Availability
All data supporting the conclusions of this manuscript are provided in the text, figures and tables.
Abbreviations
- TRPM2:
-
Transient receptor potential melastatin-2
- ADPR:
-
Adenosine diphosphate ribose
- PD:
-
Parkinson’s disease
- PARP:
-
Poly (ADP-ribose) polymerase
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- 2-APB:
-
2-Aminoethyl diphenyl borinate
- PJ-34:
-
N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-(N,N-dimethylamino) acetamide hydrochloride
- TH:
-
Tyrosine hydroxylase
- TRP:
-
Transient receptor potential
- ROS:
-
Reactive oxygen species
- NUDT9:
-
(Nucleoside diphosphate–linked moiety X)-type motif 9
- PARG:
-
Poly (ADP-ribose) glycohydrolase
- SNpc:
-
Substantia nigra pars compacta
- AP:
-
Anteroposterior
- ML:
-
Mediolateral
- DV:
-
Dorsoventral
- MDA:
-
Malondialdehyde
- ANOVA:
-
One-way analysis of variance
References
Li H (2017) TRP channel classification. Adv Exp Med Biol 976:1–8. https://doi.org/10.1007/978-94-024-1088-4_1
Thapak P, Vaidya B, Joshi HC, Singh JN, Sharma SS (2020) Therapeutic potential of pharmacological agents targeting TRP channels in CNS disorders. Pharmacol Res 159:105026. https://doi.org/10.1016/j.phrs.2020.105026
Naziroglu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36(3):355–366. https://doi.org/10.1007/s11064-010-0347-4
Ye M, Yang W, Ainscough JF, Hu XP, Li X, Sedo A, Zhang XH, Zhang X, Chen Z, Li XM, Beech DJ, Sivaprasadarao A, Luo JH, Jiang LH (2014) TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis 5(11):e1541. https://doi.org/10.1038/cddis.2014.494
Jiang LH, Yang W, Zou J, Beech DJ (2010) TRPM2 channel properties, functions and therapeutic potentials. Expert Opin Ther Targets 14(9):973–988. https://doi.org/10.1517/14728222.2010.510135
Adhya P, Sharma SS (2019) Redox TRPs in diabetes and diabetic complications: mechanisms and pharmacological modulation. Pharmacol Res 146:104271. https://doi.org/10.1016/j.phrs.2019.104271
Song K, Wang H, Kamm GB, Pohle J, Reis FC, Heppenstall P, Wende H, Siemens J (2016) The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 353(6306):1393–1398. https://doi.org/10.1126/science.aaf7537
An X, Fu Z, Mai C, Wang W, Wei L, Li D, Li C, Jiang LH (2019) Increasing the TRPM2 Channel Expression in human neuroblastoma SH-SY5Y cells augments the susceptibility to ROS-induced cell death. Cells 8 (1). https://doi.org/10.3390/cells8010028
Xu C, Macciardi F, Li PP, Yoon IS, Cooke RG, Hughes B, Parikh SV, McIntyre RS, Kennedy JL, Warsh JJ (2006) Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 141B(1):36–43. https://doi.org/10.1002/ajmg.b.30239
Akyuva Y, Naziroglu M (2020) Resveratrol attenuates hypoxia-induced neuronal cell death, inflammation and mitochondrial oxidative stress by modulation of TRPM2 channel. Sci Rep 10(1):6449. https://doi.org/10.1038/s41598-020-63577-5
Thapak P, Bishnoi M, Sharma SS (2020) Pharmacological inhibition of transient receptor potential melastatin 2 (TRPM2) channels attenuates diabetes-induced cognitive deficits in rats: a mechanistic study. Curr Neurovasc Res 17(3):249–258. https://doi.org/10.2174/1567202617666200415142211
Dietz RM, Cruz-Torres I, Orfila JE, Patsos OP, Shimizu K, Chalmers N, Deng G, Tiemeier E, Quillinan N, Herson PS (2020) Reversal of global ischemia-induced cognitive dysfunction by delayed inhibition of TRPM2 ion channels. Transl Stroke Res 11(2):254–266. https://doi.org/10.1007/s12975-019-00712-z
Abuarab N, Munsey TS, Jiang LH, Li J, Sivaprasadarao A (2017) High glucose-induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn(2+)-mediated mitochondrial fission. Sci Signal 10 (490). https://doi.org/10.1126/scisignal.aal4161
Fonfria E, Murdock PR, Cusdin FS, Benham CD, Kelsell RE, McNulty S (2006) Tissue distribution profiles of the human TRPM cation channel family. J Recept Signal Transduct Res 26(3):159–178. https://doi.org/10.1080/10799890600637506
Naziroglu M, Ozgul C, Celik O, Cig B, Sozbir E (2011) Aminoethoxydiphenyl borate and flufenamic acid inhibit Ca2+ influx through TRPM2 channels in rat dorsal root ganglion neurons activated by ADP-ribose and rotenone. J Membr Biol 241(2):69–75. https://doi.org/10.1007/s00232-011-9363-9
Vaidya B, Sharma SS (2020) Transient receptor potential channels as an emerging target for the treatment of parkinson’s disease: an insight into role of pharmacological interventions. J Frontiers in Cell Developmental Biology 8:1387. https://doi.org/10.3389/fcell.2020.584513
Nilius B, Owsianik G (2011) The transient receptor potential family of ion channels. Genome Biol 12(3):218. https://doi.org/10.1186/gb-2011-12-3-218
Fleig A, Penner R (2004) The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci 25(12):633–639. https://doi.org/10.1016/j.tips.2004.10.004
Sun Y, Sukumaran P, Selvaraj S, Cilz NI, Schaar A, Lei S, Singh BB (2018) TRPM2 Promotes neurotoxin MPP(+)/MPTP-induced cell death. Mol Neurobiol 55(1):409–420. https://doi.org/10.1007/s12035-016-0338-9
Naziroglu M, Luckhoff A (2008) A calcium influx pathway regulated separately by oxidative stress and ADP-ribose in TRPM2 channels: single channel events. Neurochem Res 33(7):1256–1262. https://doi.org/10.1007/s11064-007-9577-5
Sumoza-Toledo A, Penner R (2011) TRPM2: a multifunctional ion channel for calcium signalling. J Physiol 589(Pt 7):1515–1525. https://doi.org/10.1113/jphysiol.2010.201855
Outeiro TF, Grammatopoulos TN, Altmann S, Amore A, Standaert DG, Hyman BT, Kazantsev AG (2007) Pharmacological inhibition of PARP-1 reduces alpha-synuclein- and MPP+-induced cytotoxicity in Parkinson’s disease in vitro models. Biochem Biophys Res Commun 357(3):596–602. https://doi.org/10.1016/j.bbrc.2007.03.163
Uppalapati D, Das NR, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS (2014) Neuroprotective potential of peroxisome proliferator activated receptor-α agonist in cognitive impairment in Parkinson’s disease: Behavioral, biochemical, and PBPK profile. PPAR Res 2014. https://doi.org/10.1155/2014/753587
Das NR, Gangwal RP, Damre MV, Sangamwar AT, Sharma SS (2014) A PPAR-beta/delta agonist is neuroprotective and decreases cognitive impairment in a rodent model of Parkinson’s disease. Curr Neurovasc Res 11(2):114–124. https://doi.org/10.2174/1567202611666140318114037
Kumar P, Kaundal RK, More S, Sharma SS (2009) Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav Brain Res 197(2):398–403. https://doi.org/10.1016/j.bbr.2008.10.010
Santiago RM, Barbieiro J, Lima MM, Dombrowski PA, Andreatini R, Vital MA (2010) Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry 34(6):1104–1114. https://doi.org/10.1016/j.pnpbp.2010.06.004
Togashi K, Inada H, Tominaga M (2008) Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB). Br J Pharmacol 153(6):1324–1330. https://doi.org/10.1038/sj.bjp.0707675
Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, Ishida J, Yamamoto H, Hattori K, Matsuoka N, Mutoh S (2004) A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther 310(2):425–436. https://doi.org/10.1124/jpet.104.066944
Thapak P, Khare P, Bishnoi M, Sharma SS (2020) Neuroprotective Effect of 2-Aminoethoxydiphenyl borate (2-APB) in amyloid beta-induced memory dysfunction: a mechanistic study. Cell Mol Neurobiol. https://doi.org/10.1007/s10571-020-01012-z
Monville C, Torres EM, Dunnett SB (2006) Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods 158(2):219–223. https://doi.org/10.1016/j.jneumeth.2006.06.001
Jangra A, Datusalia AK, Khandwe S, Sharma SS (2013) Amelioration of diabetes-induced neurobehavioral and neurochemical changes by melatonin and nicotinamide: implication of oxidative stress-PARP pathway. Pharmacol Biochem Behav 114–115:43–51. https://doi.org/10.1016/j.pbb.2013.10.021
Khare P, Datusalia AK, Sharma SS (2017) Parthenolide, an NF-kappaB inhibitor ameliorates diabetes-induced behavioural deficit, neurotransmitter imbalance and neuroinflammation in type 2 diabetes rat model. Neuromolecular Med 19(1):101–112. https://doi.org/10.1007/s12017-016-8434-6
Kim BW, Koppula S, Kumar H, Park JY, Kim IW, More SV, Kim IS, Han SD, Kim SK, Yoon SH, Choi DK (2015) alpha-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 97:46–57. https://doi.org/10.1016/j.neuropharm.2015.04.037
Kaundal RK, Sharma SS (2011) GW1929: A nonthiazolidinedione PPARγ agonist, ameliorates neurological damage in global cerebral ischemic-reperfusion injury through reduction in inflammation and DNA fragmentation. Behav Brain Res 216(2):606–612. https://doi.org/10.1016/j.bbr.2010.09.001
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Bulani Y, Sharma SS (2017) Argatroban attenuates diabetic cardiomyopathy in rats by reducing fibrosis, inflammation, apoptosis, and protease-activated receptor expression. Cardiovasc Drugs Ther 31(3):255–267. https://doi.org/10.1007/s10557-017-6732-3
Negi G, Sharma SS (2015) Inhibition of IkappaB kinase (IKK) protects against peripheral nerve dysfunction of experimental diabetes. Mol Neurobiol 51(2):591–598. https://doi.org/10.1007/s12035-014-8784-8
Resham K, Sharma SS (2019) Pharmacologic inhibition of porcupine, disheveled, and beta-catenin in Wnt signaling pathway ameliorates diabetic peripheral neuropathy in rats. J Pain 20(11):1338–1352. https://doi.org/10.1016/j.jpain.2019.04.010
Chen T, Zhu J, Zhang C, Huo K, Fei Z, Jiang XF (2013) Protective effects of SKF-96365, a non-specific inhibitor of SOCE, against MPP+-induced cytotoxicity in PC12 cells: potential role of Homer1. PLoS ONE 8(1):e55601. https://doi.org/10.1371/journal.pone.0055601
Thapak P, Bishnoi M, Sharma SS (2020) Amelioration of diabetes-induced cognitive impairment by transient receptor potential vanilloid 2 (TRPV2) channel inhibitor: behavioral and mechanistic study. Neurochem Int 139:104783. https://doi.org/10.1016/j.neuint.2020.104783
Chung KK, Freestone PS, Lipski J (2011) Expression and functional properties of TRPM2 channels in dopaminergic neurons of the substantia nigra of the rat. J Neurophysiol 106(6):2865–2875. https://doi.org/10.1152/jn.00994.2010
Cruz-Torres I, Backos DS, Herson PS (2020) Characterization and optimization of the novel transient receptor potential melastatin 2 antagonist tatM2NX. Mol Pharmacol 97(2):102–111. https://doi.org/10.1124/mol.119.117549
Kuhn F, Kuhn C, Luckhoff A (2017) Different principles of ADP-ribose-mediated activation and opposite roles of the NUDT9 homology domain in the TRPM2 orthologs of man and sea anemone. Front Physiol 8:879. https://doi.org/10.3389/fphys.2017.00879
Braga R, Kouzmine I, Canteras NS, Da Cunha C (2005) Lesion of the substantia nigra, pars compacta impairs delayed alternation in a Y-maze in rats. Exp Neurol 192(1):134–141. https://doi.org/10.1016/j.expneurol.2004.11.006
Kulkarni NP, Vaidya B, Narula A, Sharma SS (2021) Neuroprotective Potential of caffeic acid phenethyl ester (CAPE) in CNS disorders: mechanistic and therapeutic insights. Curr Neuropharmacol. https://doi.org/10.2174/1570159x19666210608165509
Pallier PN, Drew CJ, Morton AJ (2009) The detection and measurement of locomotor deficits in a transgenic mouse model of Huntington’s disease are task- and protocol-dependent: influence of non-motor factors on locomotor function. Brain Res Bull 78(6):347–355. https://doi.org/10.1016/j.brainresbull.2008.10.007
O’Cearbhaill RE (2018) Using PARP inhibitors in advanced ovarian cancer. Oncology (Williston Park) 32(7):339–343
Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22:S119–S122. https://doi.org/10.1016/j.parkreldis.2015.09.004
Kim M, Cho KH, Shin MS, Lee JM, Cho HS, Kim CJ, Shin DH, Yang HJ (2014) Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int J Mol Med 33(4):870–878. https://doi.org/10.3892/ijmm.2014.1656
Inaba H, Tsukagoshi A, Kida S (2015) PARP-1 activity is required for the reconsolidation and extinction of contextual fear memory. Mol Brain 8(1):63. https://doi.org/10.1186/s13041-015-0153-7
Katerji M, Filippova M, Duerksen-Hughes P (2019) Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid Med Cell Longev 2019:1279250. https://doi.org/10.1155/2019/1279250
Yang W, Chen YH, Liu H, Qu HD (2015) Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int J Mol Med 36(5):1369–1376. https://doi.org/10.3892/ijmm.2015.2356
Yildizhan K, Naziroglu M (2020) Glutathione depletion and parkinsonian neurotoxin MPP(+)-induced TRPM2 channel activation play central roles in oxidative cytotoxicity and inflammation in microglia. Mol Neurobiol 57(8):3508–3525. https://doi.org/10.1007/s12035-020-01974-7
Johnson ME, Salvatore MF, Maiolo SA, Bobrovskaya L (2018) Tyrosine hydroxylase as a sentinel for central and peripheral tissue responses in Parkinson’s progression: evidence from clinical studies and neurotoxin models. Prog Neurobiol 165–167:1–25. https://doi.org/10.1016/j.pneurobio.2018.01.002
Nagatsu T, Nakashima A, Ichinose H, Kobayashi K (2019) Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J Neural Transm (Vienna) 126(4):397–409. https://doi.org/10.1007/s00702-018-1903-3
Ratnam M, Chan J, Lesani N, Sidorova-Darmos E, Eubanks JH, Aarts MM (2018) mRNA expression of transient receptor potential melastatin (TRPM) channels 2 and 7 in perinatal brain development. Int J Dev Neurosci 69:23–31. https://doi.org/10.1016/j.ijdevneu.2018.05.008
Fonfria E, Marshall IC, Boyfield I, Skaper SD, Hughes JP, Owen DE, Zhang W, Miller BA, Benham CD, McNulty S (2005) Amyloid beta-peptide(1–42) and hydrogen peroxide-induced toxicity are mediated by TRPM2 in rat primary striatal cultures. J Neurochem 95(3):715–723. https://doi.org/10.1111/j.1471-4159.2005.03396.x
Colton CK, Zhu MX (2007) 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb Exp Pharmacol 179:173–187. https://doi.org/10.1007/978-3-540-34891-7_10
Negi G, Kumar A, Sharma SS (2010) Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun 391(1):102–106. https://doi.org/10.1016/j.bbrc.2009.11.010
Djillani A, Nüße O (1843) Dellis O (2014) Characterization of novel store-operated calcium entry effectors. Biochim Biophys Acta 10:2341–2347. https://doi.org/10.1016/j.bbamcr.2014.03.012
Barbiero JK, Santiago R, Tonin FS, Boschen S, da Silva LM, Werner MF, da Cunha C, Lima MM, Vital MA (2014) PPAR-α agonist fenofibrate protects against the damaging effects of MPTP in a rat model of Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 53:35–44. https://doi.org/10.1016/j.pnpbp.2014.02.009
Funding
The present study was supported by financial support from start-up grant (R-12020/2017-HR) Department of Health Research, Ministry of Health and Family Welfare, Government of India. Also, authors received financial support from the National Institute of Pharmaceutical Education and Research, S.A.S. Nagar, Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India to carry out this work.
Author information
Authors and Affiliations
Contributions
BV: data curation; formal analysis; investigation; methodology; validation; visualization; writing—original draft; writing—review and editing. HK: investigation; data curation; formal analysis; methodology; visualization. PT: western bloting investigation and analysis; visualization. SSS: conceptualization; resources; supervision; writing—final review and editing. JNS: conceptualization; data curation; funding acquisition; project administration; resources; supervision; validation; visualization; roles/writing—original draft; review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Research involving Human Participants and/or Animals
Experiments were carried out in accordance with the Committee for the Purpose of Control and Supervision on Experiments on Animals, Government of India; and after approval of Institutional Animal Ethics Committee of National Institute of Pharmaceutical Education and Research, SAS Nagar, Punjab, India (IAEC/18/19 and IAEC/ 17/25).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors have contributed equally to this work and share first authorship
Supplementary Information
Below is the link to the electronic supplementary material.
12035_2021_2711_MOESM1_ESM.jpg
Supplementary file1 Supplementary Fig 1: Fura-2AM assay for the measurement of intracellular calcium influx to study the effect of 2-APB on calcium influx on SH-SY5Y cells. (JPG 2433 KB)
12035_2021_2711_MOESM2_ESM.jpg
Supplementary file2 Supplementary Fig 2: GSH estimation carried out in the (A) Mid-Brain (B) Hippocampus (C) Striatum (D) Cortex. (JPG 3172 KB)
Rights and permissions
About this article
Cite this article
Vaidya, B., Kaur, H., Thapak, P. et al. Pharmacological Modulation of TRPM2 Channels via PARP Pathway Leads to Neuroprotection in MPTP-induced Parkinson’s Disease in Sprague Dawley Rats. Mol Neurobiol 59, 1528–1542 (2022). https://doi.org/10.1007/s12035-021-02711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02711-4