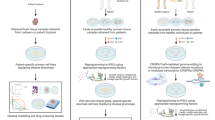
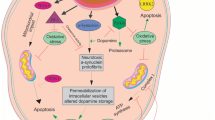
Abstract
Neurological disorders are primarily diseases with sophisticated etiology that are always refractory and recrudescent. The major obstruction to effective therapies for neurological disorders is the poor understanding of their pathogenic mechanisms. CRISPR-Cas9 technology, which allows precise and effective gene editing in almost any cell type and organism, is accelerating the pace of basic biological research. An increasing number of groups are focusing on uncovering the molecular mechanisms of neurological disorders and developing novel therapies using the CRISPR-Cas9 system. This review highlights the application of CRISPR-Cas9 technology in the treatment of neurological disorders, including Alzheimer’s disease, amyotrophic lateral sclerosis and/or frontotemporal dementia, Duchenne muscular dystrophy, Dravet syndrome, epilepsy, Huntington’s disease, and Parkinson’s disease. Hopefully, it will improve our understanding of neurological disorders and give insights into future treatments for neurological disorders.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Misra MK, Damotte V, Hollenbach JA (2018) The immunogenetics of neurological disease Immunology 153(4):399–414
Group GBDNDC (2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 16(11):877–897
Feigin VL, Vos T, Nichols E, Owolabi MO, Carroll WM, Dichgans M, Deuschl G, Parmar P, Brainin M, Murray C (2020) The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 19(3):255–265
Pena SA, Iyengar R, Eshraghi RS, Bencie N, Mittal J, Aljohani A, Mittal R, Eshraghi AA (2020) Gene therapy for neurological disorders: challenges and recent advancements. J Drug Target 28(2):111–128
Huang H, Sharma HS, Chen L, Saberi H, Mao G (2019) 2018 Yearbook of Neurorestoratology. Journal of Neurorestoratology 7(1):8–17
Huang H, Chen L, Mao G, Bach J, Xue Q, Han F, Guo X, Otom A, Chernykh E, Alvarez E et al (2020) The 2019 yearbook of Neurorestoratology. Journal of Neurorestoratology 8(1):1–11
Huang H, Chen L, Mao G, Sharma H (2020) Clinical neurorestorative cell therapies: developmental process, current state, and future prospective. Journal of Neurorestoratology 8(2):61–82
Huang H, Chen L, Chopp M, Young W, Bach JR, He X, Sarnowaska A, Xue M, Zhao R, Shetty A et al (2021) The 2020 Yearbook of Neurorestoratology. Journal of Neurorestoratology 9(1):1–12
Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G (2018) Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 135(3):311–336
Daneman R, Prat A (2015) The blood-brain barrier. Cold Spring Harb Perspect Biol 7(1):a020412
Benz F, Liebner S (2020) Structure and function of the blood-brain barrier (BBB). In: Handbook of experimental pharmacology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp 1–29
Pankevich DE, Altevogt BM, Dunlop J, Gage FH, Hyman SE (2014) Improving and accelerating drug development for nervous system disorders. Neuron 84(3):546–553
Grissa I, Vergnaud G, Pourcel C (2007) The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinformatics 8:172
Kunin V, Sorek R, Hugenholtz P (2007) Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol 8(4):R61
Sorek R, Kunin V, Hugenholtz P (2008) CRISPR–a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol 6(3):181–186
Horvath P, Barrangou R (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327(5962):167–170
Marraffini LA, Sontheimer EJ (2010) CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat Rev Genet 11(3):181–190
Jansen R, Embden JD, Gaastra W, Schouls LM (2002) Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol 43(6):1565–1575
Deveau H, Garneau JE, Moineau S (2010) CRISPR/Cas system and its role in phage-bacteria interactions. Annu Rev Microbiol 64:475–493
Haft DH, Selengut J, Mongodin EF, Nelson KE (2005) A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Computational Biology 1(6):e60
Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF et al (2011) Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol 9(6):467–477
Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH et al (2015) An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol 13(11):722–736
Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E (2005) Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol 60(2):174–182
Pourcel C, Salvignol G, Vergnaud G (2005) CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 151(Pt 3):653–663
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315(5819):1709–1712
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339(6121):819–823
Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, Lim WA (2013) Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152(5):1173–1183
Larson MH, Gilbert LA, Wang X, Lim WA, Weissman JS, Qi LS (2013) CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat Protoc 8(11):2180–2196
Dominguez AA, Lim WA, Qi LS (2016) Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol 17(1):5–15
Gilbert LA, Larson MH, Morsut L, Liu Z, Brar GA, Torres SE, Stern-Ginossar N, Brandman O, Whitehead EH, Doudna JA et al (2013) CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154(2):442–451
Yu S, Price MA, Wang Y, Liu Y, Guo Y, Ni X, Rosser SJ, Bi C, Wang M (2020) CRISPR-dCas9 mediated cytosine deaminase base editing in bacillus subtilis. ACS Synth Biol 9(7):1781–1789
Giau VV, Lee H, Shim KH, Bagyinszky E, An SSA (2018) Genome-editing applications of CRISPR-Cas9 to promote in vitro studies of Alzheimer’s disease. Clin Interv Aging 13:221–233
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Selkoe DJ (2001) Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev 81(2):741–766
Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L (1992) A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1(5):345–347
Gyorgy B, Loov C, Zaborowski MP, Takeda S, Kleinstiver BP, Commins C, Kastanenka K, Mu D, Volak A, Giedraitis V et al (2018) CRISPR/Cas9 mediated disruption of the Swedish APP allele as a therapeutic approach for early-onset Alzheimer’s disease. Mol Ther Nucleic Acids 11:429–440
Tomita T, Maruyama K, Saido TC, Kume H, Shinozaki K, Tokuhiro S, Capell A, Walter J, Grunberg J, Haass C et al (1997) The presenilin 2 mutation (N141I) linked to familial Alzheimer disease (Volga German families) increases the secretion of amyloid beta protein ending at the 42nd (or 43rd) residue. Proc Natl Acad Sci U S A 94(5):2025–2030
Ortiz-Virumbrales M, Moreno CL, Kruglikov I, Marazuela P, Sproul A, Jacob S, Zimmer M, Paull D, Zhang B, Schadt EE et al (2017) CRISPR/Cas9-correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer’s PSEN2 (N141I) neurons. Acta Neuropathol Commun 5(1):77
Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R (2018) Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation 15(1):276
Thangavel R, Stolmeier D, Yang X, Anantharam P, Zaheer A (2012) Expression of glia maturation factor in neuropathological lesions of Alzheimer’s disease. Neuropathol Appl Neurobiol 38(6):572–581
Raikwar SP, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, Kempuraj D, Zaheer SA, Iyer SS, Zaheer A (2019) Targeted gene editing of glia maturation factor in microglia: a novel Alzheimer’s disease therapeutic target. Mol Neurobiol 56(1):378–393
Butterfield DA, Boyd-Kimball D (2004) Amyloid beta-peptide(1–42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol 14(4):426–432
Hampel H, Vassar R, De Strooper B, Hardy J, Willem M, Singh N, Zhou J, Yan R, Vanmechelen E, De Vos A et al (2021) The β-secretase BACE1 in Alzheimer’s disease. Biol Psychiatry 89(8):745–756
Park H, Oh J, Shim G, Cho B, Chang Y, Kim S, Baek S, Kim H, Shin J, Choi H et al (2019) In vivo neuronal gene editing via CRISPR-Cas9 amphiphilic nanocomplexes alleviates deficits in mouse models of Alzheimer’s disease. Nat Neurosci 22(4):524–528
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955
Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W, Shaw PJ, Simmons Z, van den Berg LH (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Primers 3:17071
Wang WY, Pan L, Su SC, Quinn EJ, Sasaki M, Jimenez JC, Mackenzie IR, Huang EJ, Tsai LH (2013) Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat Neurosci 16(10):1383–1391
Wang H, Guo W, Mitra J, Hegde PM, Vandoorne T, Eckelmann BJ, Mitra S, Tomkinson AE, Van Den Bosch L, Hegde ML (2018) Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in amyotrophic lateral sclerosis. Nat Commun 9(1):3683
Park KH (2015) Mechanisms of muscle denervation in aging: insights from a mouse model of amyotrophic lateral sclerosis. Aging Dis 6(5):380–389
Gurney ME (1994) Transgenic-mouse model of amyotrophic lateral sclerosis. N Engl J Med 331(25):1721–1722
Gaj T, Ojala DS, Ekman FK, Byrne LC, Limsirichai P, Schaffer DV (2017) In vivo genome editing improves motor function and extends survival in a mouse model of ALS. Sci Adv 3(12):eaar3952
Duan W, Guo M, Yi L, Liu Y, Li Z, Ma Y, Zhang G, Liu Y, Bu H, Song X et al (2020) The deletion of mutant SOD1 via CRISPR/Cas9/sgRNA prolongs survival in an amyotrophic lateral sclerosis mouse model. Gene Ther 27(3–4):157–169
Wang L, Yi F, Fu L, Yang J, Wang S, Wang Z, Suzuki K, Sun L, Xu X, Yu Y et al (2017) CRISPR/Cas9-mediated targeted gene correction in amyotrophic lateral sclerosis patient iPSCs. Protein Cell 8(5):365–378
Bang J, Spina S, Miller BL (2015) Frontotemporal dementia. Lancet 386(10004):1672–1682
Clayton EL, Mizielinska S, Edgar JR, Nielsen TT, Marshall S, Norona FE, Robbins M, Damirji H, Holm IE, Johannsen P et al (2015) Frontotemporal dementia caused by CHMP2B mutation is characterised by neuronal lysosomal storage pathology. Acta Neuropathol 130(4):511–523
Zhang Y, Schmid B, Nikolaisen NK, Rasmussen MA, Aldana BI, Agger M, Calloe K, Stummann TC, Larsen HM, Nielsen TT et al (2017) Patient iPSC-derived neurons for disease modeling of frontotemporal dementia with mutation in CHMP2B. Stem Cell Reports 8(3):648–658
Balendra R, Isaacs AM (2018) C9orf72-mediated ALS and FTD: multiple pathways to disease. Nat Rev Neurol 14(9):544–558
Lopez-Gonzalez R, Yang D, Pribadi M, Kim TS, Krishnan G, Choi SY, Lee S, Coppola G, Gao FB (2019) Partial inhibition of the overactivated Ku80-dependent DNA repair pathway rescues neurodegeneration in C9ORF72-ALS/FTD. Proc Natl Acad Sci U S A 116(19):9628–9633
Ash PE, Bieniek KF, Gendron TF, Caulfield T, Lin WL, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW 3rd, Rademakers R et al (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron 77(4):639–646
Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C et al (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science 339(6125):1335–1338
Zu T, Liu Y, Banez-Coronel M, Reid T, Pletnikova O, Lewis J, Miller TM, Harms MB, Falchook AE, Subramony SH et al (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc Natl Acad Sci U S A 110(51):E4968-4977
Krishnan G, Zhang Y, Gu Y, Kankel MW, Gao FB, Almeida S (2020) CRISPR deletion of the C9ORF72 promoter in ALS/FTD patient motor neurons abolishes production of dipeptide repeat proteins and rescues neurodegeneration. Acta Neuropathol 140(1):81–84
Ababneh NA, Scaber J, Flynn R, Douglas A, Barbagallo P, Candalija A, Turner MR, Sims D, Dafinca R, Cowley SA et al (2020) Correction of amyotrophic lateral sclerosis related phenotypes in induced pluripotent stem cell-derived motor neurons carrying a hexanucleotide expansion mutation in C9orf72 by CRISPR/Cas9 genome editing using homology-directed repair. Hum Mol Genet 29(13):2200–2217
Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM (1986) Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 323(6089):646–650
Hoffman EP, Brown RH Jr, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51(6):919–928
Roberts RG, Coffey AJ, Bobrow M, Bentley DR (1993) Exon structure of the human dystrophin gene. Genomics 16(2):536–538
Hotta A (2015) Genome editing gene therapy for duchenne muscular dystrophy. J Neuromuscul Dis 2(4):343–355
Chamberlain JR, Chamberlain JS (2017) Progress toward gene therapy for duchenne muscular dystrophy. Mol Ther 25(5):1125–1131
Gee P, Xu H, Hotta A (2017) Cellular reprogramming, genome editing, and alternative CRISPR Cas9 technologies for precise gene therapy of duchenne muscular dystrophy. Stem Cells Int 2017:8765154
Bulfield G, Siller WG, Wight PA, Moore KJ (1984) X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A 81(4):1189–1192
Xu L, Park KH, Zhao L, Xu J, El Refaey M, Gao Y, Zhu H, Ma J, Han R (2016) CRISPR-mediated genome editing restores dystrophin expression and function in mdx mice. Mol Ther 24(3):564–569
Tabebordbar M, Zhu K, Cheng JKW, Chew WL, Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA et al (2016) In vivo gene editing in dystrophic mouse muscle and muscle stem cells. Science 351(6271):407–411
Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan X, Ran FA, Yan WX et al (2016) In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science 351(6271):403–407
Long C, Amoasii L, Mireault AA, McAnally JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby R, Olson EN (2016) Postnatal genome editing partially restores dystrophin expression in a mouse model of muscular dystrophy. Science 351(6271):400–403
El Refaey M, Xu L, Gao Y, Canan BD, Adesanya TMA, Warner SC, Akagi K, Symer DE, Mohler PJ, Ma J et al (2017) In vivo genome editing restores dystrophin expression and cardiac function in dystrophic mice. Circ Res 121(8):923–929
Hakim CH, Wasala NB, Nelson CE, Wasala LP, Yue Y, Louderman JA, Lessa TB, Dai A et al (2018) AAV CRISPR editing rescues cardiac and muscle function for 18 months in dystrophic mice. JCI Insight 3(23):e124297
Nance ME, Shi R, Hakim CH, Wasala NB, Yue Y, Pan X, Zhang T, Robinson CA, Duan SX, Yao G et al (2019) AAV9 edits muscle stem cells in normal and dystrophic adult mice. Mol Ther 27(9):1568–1585
Matre PR, Mu X, Wu J, Danila D, Hall MA, Kolonin MG, Darabi R, Huard J (2019) CRISPR/Cas9-Based dystrophin restoration reveals a novel role for dystrophin in bioenergetics and stress resistance of muscle progenitors. Stem Cells 37(12):1615–1628
Xu L, Lau YS, Gao Y, Li H, Han R (2019) Life-long AAV-mediated crispr genome editing in dystrophic heart improves cardiomyopathy without causing serious lesions in mdx mice. Mol Ther 27(8):1407–1414
Nelson CE, Wu Y, Gemberling MP, Oliver ML, Waller MA, Bohning JD, Robinson-Hamm JN, Bulaklak K, Castellanos Rivera RM, Collier JH et al (2019) Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat Med 25(3):427–432
Kwon JB, Ettyreddy AR, Vankara A, Bohning JD, Devlin G, Hauschka SD, Asokan A, Gersbach CA (2020) In vivo gene editing of muscle stem cells with adeno-associated viral vectors in a mouse model of duchenne muscular dystrophy. Mol Ther Methods Clin Dev 19:320–329
Petkova MV, Stantzou A, Morin A, Petrova O, Morales-Gonzalez S, Seifert F, Bellec-Dyevre J, Manoliu T, Goyenvalle A, Garcia L et al (2020) Live-imaging of revertant and therapeutically restored dystrophin in the Dmd(EGFP-mdx) mouse model for Duchenne muscular dystrophy. Neuropathol Appl Neurobiol 46(6):602–614
Long CZ, McAnally JR, Shelton JM, Mireault AA, Bassel-Duby R, Olson EN (2014) Prevention of muscular dystrophy in mice by CRISPR/Cas9-mediated editing of germline DNA. Science 345(6201):1184–1188
Zhu P, Wu F, Mosenson J, Zhang H, He TC, Wu WS (2017) CRISPR/Cas9-mediated genome editing corrects dystrophin mutation in skeletal muscle stem cells in a mouse model of muscle dystrophy. Mol Ther Nucleic Acids 7:31–41
Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C et al (2017) Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair. Nat Biomed Eng 1:889–901
Min YL, Chemello F, Li H, Rodriguez-Caycedo C, Sanchez-Ortiz E, Mireault AA, McAnally JR, Shelton JM, Zhang Y, Bassel-Duby R et al (2020) Correction of three prominent mutations in mouse and human models of duchenne muscular dystrophy by single-cut genome editing. Mol Ther 28(9):2044–2055
Zhang Y, Li H, Min YL, Sanchez-Ortiz E, Huang J, Mireault AA, Shelton JM, Kim J, Mammen PPA, Bassel-Duby R et al (2020) Enhanced CRISPR-Cas9 correction of Duchenne muscular dystrophy in mice by a self-complementary AAV delivery system. Sci Adv 6(8):eaay6812
Amoasii L, Long C, Li H, Mireault AA, Shelton JM, Sanchez-Ortiz E, McAnally JR, Bhattacharyya S et al (2017) Single-cut genome editing restores dystrophin expression in a new mouse model of muscular dystrophy. Sci Transl Med 9(418):eaan8081
Long C, Li H, Tiburcy M, Rodriguez-Caycedo C, Kyrychenko V, Zhou H, Zhang Y, Min YL, Shelton JM, Mammen PPA et al (2018) Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci Adv 4(1):eaap9004
Yuan J, Ma Y, Huang T, Chen Y, Peng Y, Li B, Li J, Zhang Y, Song B, Sun X et al (2018) Genetic modulation of RNA splicing with a CRISPR-guided cytidine deaminase. Mol Cell 72(2):380-394 e7
Min YL, Li H, Rodriguez-Caycedo C, Mireault AA, Huang J, Shelton JM, McAnally JR, Amoasii L, Mammen PPA, Bassel-Duby R et al (2019) CRISPR-Cas9 corrects Duchenne muscular dystrophy exon 44 deletion mutations in mice and human cells. Sci Adv 5(3):eaav4324
Ousterout DG, Kabadi AM, Thakore PI, Majoros WH, Reddy TE, Gersbach CA (2015) Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat Commun 6:6244
Iyombe-Engembe JP, Ouellet DL, Barbeau X, Rousseau J, Chapdelaine P, Lague P, Tremblay JP (2016) Efficient restoration of the dystrophin gene reading frame and protein structure in DMD myoblasts using the CinDel method. Mol Ther Nucleic Acids 5:e283
Wojtal D, Kemaladewi DU, Malam Z, Abdullah S, Wong TW, Hyatt E, Baghestani Z, Pereira S, Stavropoulos J, Mouly V et al (2016) Spell checking nature: versatility of CRISPR/Cas9 for developing treatments for inherited disorders. Am J Hum Genet 98(1):90–101
Lattanzi A, Duguez S, Moiani A, Izmiryan A, Barbon E, Martin S, Mamchaoui K, Mouly V, Bernardi F, Mavilio F et al (2017) Correction of the exon 2 duplication in DMD myoblasts by a single CRISPR/Cas9 system. Mol Ther Nucleic Acids 7:11–19
Kyrychenko V, Kyrychenko S, Tiburcy M, Shelton JM, Long C, Schneider JW, Zimmermann WH, Bassel-Duby R et al (2017) Functional correction of dystrophin actin binding domain mutations by genome editing. JCI Insight 2(18):e95918
Duchene BL, Cherif K, Iyombe-Engembe JP, Guyon A, Rousseau J, Ouellet DL, Barbeau X, Lague P, Tremblay JP (2018) CRISPR-induced deletion with SaCas9 restores dystrophin expression in dystrophic models in vitro and in vivo. Mol Ther 26(11):2604–2616
Brescia M, Janssen JM, Liu J, Goncalves M (2020) High-capacity adenoviral vectors permit robust and versatile testing of DMD gene repair tools and strategies in human cells. Cells 9(4):869
Li HL, Fujimoto N, Sasakawa N, Shirai S, Ohkame T, Sakuma T, Tanaka M, Amano N, Watanabe A, Sakurai H et al (2015) Precise correction of the dystrophin gene in duchenne muscular dystrophy patient induced pluripotent stem cells by TALEN and CRISPR-Cas9. Stem Cell Reports 4(1):143–154
Young CS, Hicks MR, Ermolova NV, Nakano H, Jan M, Younesi S, Karumbayaram S, Kumagai-Cresse C, Wang D, Zack JA et al (2016) A single CRISPR-Cas9 deletion strategy that targets the majority of DMD patients restores dystrophin function in hiPSC-derived muscle cells. Cell Stem Cell 18(4):533–540
Maggio I, Liu J, Janssen JM, Chen X, Goncalves MA (2016) Adenoviral vectors encoding CRISPR/Cas9 multiplexes rescue dystrophin synthesis in unselected populations of DMD muscle cells. Sci Rep 6:37051
Xiang X, Zhao X, Pan X, Dong Z, Yu J, Li S, Liang X, Han P, Qu K, Jensen JB et al (2021) Efficient correction of Duchenne muscular dystrophy mutations by SpCas9 and dual gRNAs. Mol Ther Nucleic Acids 24:403–415
Lyu P, Yoo KW, Yadav MK, Atala A, Aartsma-Rus A, Putten MV, Duan D, Lu B (2020) Sensitive and reliable evaluation of single-cut sgRNAs to restore dystrophin by a GFP-reporter assay. PLoS One 15(9):e0239468
Marini C, Scheffer IE, Nabbout R, Suls A, De Jonghe P, Zara F, Guerrini R (2011) The genetics of Dravet syndrome. Epilepsia 52(Suppl 2):24–29
Scheffer IE, Nabbout R (2019) SCN1A-related phenotypes: epilepsy and beyond. Epilepsia 60(Suppl 3):S17–S24
Mei D, Cetica V, Marini C, Guerrini R (2019) Dravet syndrome as part of the clinical and genetic spectrum of sodium channel epilepsies and encephalopathies. Epilepsia 60(Suppl 3):S2–S7
Ito S, Ogiwara I, Yamada K, Miyamoto H, Hensch TK, Osawa M, Yamakawa K (2013) Mouse with Nav1.1 haploinsufficiency, a model for Dravet syndrome, exhibits lowered sociability and learning impairment. Neurobiol Dis 49:29–40
Colasante G, Lignani G, Brusco S, Di Berardino C, Carpenter J, Giannelli S, Valassina N, Bido S, Ricci R, Castoldi V et al (2020) dCas9-based scn1a gene activation restores inhibitory interneuron excitability and attenuates seizures in Dravet syndrome mice. Mol Ther 28(1):235–253
Thijs RD, Surges R, O’Brien TJ, Sander JW (2019) Epilepsy in adults. Lancet 393(10172):689–701
Robbins CA, Tempel BL (2012) Kv1.1 and Kv1.2: similar channels, different seizure models. Epilepsia 53(Suppl 1):134–141
D’Adamo MC, Liantonio A, Rolland JF, Pessia M, Imbrici P (2020) Kv1.1 Channelopathies: pathophysiological mechanisms and therapeutic approaches. Int J Mol Sci 21(8):2935
Colasante G, Qiu Y, Massimino L, Di Berardino C, Cornford JH, Snowball A, Weston M, Jones SP, Giannelli S, Lieb A et al (2020) In vivo CRISPRa decreases seizures and rescues cognitive deficits in a rodent model of epilepsy. Brain 143(3):891–905
Tabrizi SJ, Ghosh R, Leavitt BR (2019) Huntingtin lowering strategies for disease modification in Huntington’s disease. Neuron 102(4):899
Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R et al (2015) Huntington disease Nat Rev Dis Primers 1:15005
Shin JW, Kim KH, Chao MJ, Atwal RS, Gillis T, MacDonald ME, Gusella JF, Lee JM (2016) Permanent inactivation of Huntington’s disease mutation by personalized allele-specific CRISPR/Cas9. Hum Mol Genet 25(20):4566–4576
Kolli N, Lu M, Maiti P, Rossignol J, Dunbar GL (2017) CRISPR-Cas9 mediated gene-silencing of the mutant huntingtin gene in an in vitro model of Huntington’s disease. Int J Mol Sci 18(4):754
Monteys AM, Ebanks SA, Keiser MS, Davidson BL (2017) CRISPR/Cas9 editing of the mutant huntingtin allele in vitro and in vivo. Mol Ther 25(1):12–23
Yang S, Chang R, Yang H, Zhao T, Hong Y, Kong HE, Sun X, Qin Z, Jin P, Li S et al (2017) CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington’s disease. J Clin Invest 127(7):2719–2724
Ekman FK, Ojala DS, Adil MM, Lopez PA, Schaffer DV, Gaj T (2019) CRISPR-Cas9-mediated genome editing increases lifespan and improves motor deficits in a Huntington’s disease mouse model. Mol Ther Nucleic Acids 17:829–839
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912
Soman SK, Bazala M, Keatinge M, Bandmann O, Kuznicki J (2019) Restriction of mitochondrial calcium overload by mcu inactivation renders a neuroprotective effect in zebrafish models of Parkinson’s disease. Biol Open 8(10):bio044347
Kolli N, Lu M, Maiti P, Rossignol J, Dunbar GL (2018) Application of the gene editing tool, CRISPR-Cas9, for treating neurodegenerative diseases. Neurochem Int 112:187–196
Safari F, Hatam G, Behbahani AB, Rezaei V, Barekati-Mowahed M, Petramfar P, Khademi F (2020) CRISPR system: a high-throughput toolbox for research and treatment of Parkinson’s disease. Cell Mol Neurobiol 40(4):477–493
Wullner U, Kaut O, deBoni L, Piston D, Schmitt I (2016) DNA methylation in Parkinson’s disease. J Neurochem 139(Suppl 1):108–120
Kantor B, Tagliafierro L, Gu J, Zamora ME, Ilich E, Grenier C, Huang ZY, Murphy S, Chiba-Falek O (2018) Downregulation of SNCA expression by targeted editing of DNA methylation: a potential strategy for precision therapy in PD. Mol Ther 26(11):2638–2649
Homayoun H (2018) Parkinson disease. Ann Intern Med 169(5): ITC33–48.
Li H, Yahaya BH, Ng WH, Yusoff NM, Lin J (2019) Conditioned medium of human menstrual blood-derived endometrial stem cells protects against MPP(+)-induced cytotoxicity in vitro. Front Mol Neurosci 12:80
Chen Y, Xiong M, Dong Y, Haberman A, Cao J, Liu H, Zhou W, Zhang SC (2016) Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson’s disease. Cell Stem Cell 18(6):817–826
Bayarsaikhan E, Bayarsaikhan D, Lee J, Son M, Oh S, Moon J, Park HJ, Roshini A, Kim SU, Song BJ et al (2015) Microglial AGE-albumin is critical for neuronal death in Parkinson’s disease: a possible implication for theranostics. Int J Nanomedicine 10(Spec Iss):281–292
Lee J, Bayarsaikhan D, Arivazhagan R, Park H, Lim B, Gwak P, Jeong GB, Lee J, Byun K, Lee B (2019) CRISPR/Cas9 edited sRAGE-MSCs protect neuronal death in Parkinsons disease Model. Int J Stem Cells 12(1):114–124
Barodia SK, Creed RB, Goldberg MS (2017) Parkin and PINK1 functions in oxidative stress and neurodegeneration. Brain Res Bull 133:51–59
Liao Y, Dong Y, Cheng J (2017) The function of the mitochondrial calcium uniporter in neurodegenerative disorders. Int J Mol Sci 18(2):248
Zhang XH, Tee LY, Wang XG, Huang QS, and Yang SH (2015) Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol Ther Nucleic Acids 4: e264.
Fu Y, Sander JD, Reyon D, Cascio VM, Joung JK (2014) Improving CRISPR-Cas nuclease specificity using truncated guide RNAs. Nat Biotechnol 32(3):279–284
Hajiahmadi Z, Movahedi A, Wei H, Li D, Orooji Y, Ruan H, Zhuge Q (2019) Strategies to increase on-target and reduce off-target effects of the CRISPR/Cas9 system in plants. Int J Mol Sci 20(15):3719
Ren X, Yang Z, Xu J, Sun J, Mao D, Hu Y, Yang SJ, Qiao HH, Wang X, Hu Q et al (2014) Enhanced specificity and efficiency of the CRISPR/Cas9 system with optimized sgRNA parameters in Drosophila. Cell Rep 9(3):1151–1162
Ren F, Ren C, Zhang Z, Duan W, Lecourieux D, Li S, Liang Z (2019) Efficiency optimization of CRISPR/Cas9-mediated targeted mutagenesis in grape. Front Plant Sci 10:612
Lin Y, Cradick TJ, Brown MT, Deshmukh H, Ranjan P, Sarode N, Wile BM, Vertino PM, Stewart FJ, Bao G (2014) CRISPR/Cas9 systems have off-target activity with insertions or deletions between target DNA and guide RNA sequences. Nucleic Acids Res 42(11):7473–7485
Manghwar H, Li B, Ding X, Hussain A, Lindsey K, Zhang X, Jin S (2020) CRISPR/Cas Systems in genome editing: methodologies and tools for sgRNA design, off-target evaluation, and strategies to mitigate off-target effects. Adv Sci (Weinh) 7(6):1902312
Yin H, Song CQ, Suresh S, Kwan SY, Wu Q, Walsh S, Ding J, Bogorad RL, Zhu LJ, Wolfe SA et al (2018) Partial DNA-guided Cas9 enables genome editing with reduced off-target activity. Nat Chem Biol 14(3):311–316
Jia K, Lu Z, Zhou F, Xiong Z, Zhang R, Liu Z, Ma Y, He L, Li C, Zhu Z et al (2019) Multiple sgRNAs facilitate base editing-mediated i-stop to induce complete and precise gene disruption. Protein Cell 10(11):832–839
Jang DE, Lee JY, Lee JH, Koo OJ, Bae HS, Jung MH, Bae JH, Hwang WS, Chang YJ, Lee YH et al (2018) Multiple sgRNAs with overlapping sequences enhance CRISPR/Cas9-mediated knock-in efficiency. Exp Mol Med 50(4):1–9
Kleinstiver BP, Pattanayak V, Prew MS, Tsai SQ, Nguyen NT, Zheng Z, Joung JK (2016) High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects. Nature 529(7587):490–495
Slaymaker IM, Gao L, Zetsche B, Scott DA, Yan WX, Zhang F (2016) Rationally engineered Cas9 nucleases with improved specificity. Science 351(6268):84–88
Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA (2017) Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature 550(7676):407–410
Naeem M, Majeed S, Hoque MZ, Ahmad I (2020) Latest developed strategies to minimize the off-target effects in CRISPR-Cas-mediated genome editing. Cells 9(7):1608
Yang Y, Xu J, Ge S, and Lai L (2021) CRISPR/Cas: advances, limitations, and applications for precision cancer research. Front Med (Lausanne) 8: 649896.
Han HA, Pang JKS, Soh BS (2020) Mitigating off-target effects in CRISPR/Cas9-mediated in vivo gene editing. J Mol Med (Berl) 98(5):615–632
Li Y, Glass Z, Huang M, Chen ZY, and Xu Q (2020) Ex vivo cell-based CRISPR/Cas9 genome editing for therapeutic applications. Biomaterials 234: 119711.
Borbolla-Jimenez FV, Del Prado-Audelo ML, Cisneros B, Caballero-Floran IH, Leyva-Gomez G, Magana JJ (2021) New perspectives of gene therapy on polyglutamine spinocerebellar ataxias: from molecular targets to novel nanovectors. Pharmaceutics 13(7):1018
Yan J, Kang DD, Dong Y (2021) Harnessing lipid nanoparticles for efficient CRISPR delivery. Biomater Sci 9(18):6001–6011
Vetchinova AS, Simonova VV, Novosadova EV, Manuilova ES, Nenasheva VV, Tarantul VZ, Grivennikov IA, Khaspekov LG, Illarioshkin SN (2018) Cytogenetic analysis of the results of genome editing on the cell model of Parkinson’s disease. Bull Exp Biol Med 165(3):378–381
Kosicki M, Tomberg K, Bradley A (2018) Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol 36(8):765–771
Acknowledgements
We would like to thank Dr. Zhenyu Hao (Zhengzhou University of Light Industry) for his comments and suggestions.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81801127, 81771226, 81800792, U1804186), Natural Science Foundation of Henan Province for Distinguished Young Scholars (202300410307), Science and Technology Innovative Research Team in Higher Educational Institutions of Henan Province (19IRTSTHN003), Henan Province Science and Technology Project (212102310215), Major Cultivation Plan of Scientific and Technological Achievements from Natural Science Class of Xinxiang Medical University (20172DCG-03), Key Scientific Research Program of Higher Education in Henan Province (21A180023), Xinxiang Scientific and Technological Project (GG2019009), Doctoral Scientific Research Program Foundation of Xinxiang Medical University (XYBSKYZZ201523), and Open Program of Henan Key Lab of Biological Psychiatry (ZDSYS2015004).
Author information
Authors and Affiliations
Contributions
Both GLH and LJT conceived the idea for the article. Both GLH and HYW searched the literature. GLH wrote the manuscript. LSX created the figures with Adobe Illustrator. YCQ, DJ, and LH revised the manuscript. LJT supervised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, L., Han, Y., Yang, C. et al. CRISPR-Cas9-Mediated Gene Therapy in Neurological Disorders. Mol Neurobiol 59, 968–982 (2022). https://doi.org/10.1007/s12035-021-02638-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02638-w