Abstract
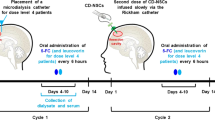
Widespread investigation has revealed the promising ability of suicidal genes in the treatment of glioma tumors; nevertheless, promoting their effects relies on the ability to apply suitable vehicles and techniques. In this study, the safety and feasibility of using bone marrow–derived mesenchymal stem cells (MSCs) in combination with prodrug for treatment of patients with primary and secondary glioblastoma multiform (GBM) was assessed. Five GBM patients were recruited. Following gross total resection of the tumor and adjuvant radiotherapy and chemotherapy, intracerebral injection of autologous MSCs transduced with lentivirus containing herpes simplex virus thymidine kinase (HSV-TK) was performed followed by intravenous administration of ganciclovir for 2 weeks. The treatment was well tolerated by all patients. Mild-to-moderate fever, headache, and cerebrospinal fluid leukocytosis were evident in three, two, and one patient, respectively. The progression-free survival (PFS) and overall survival (OS) of patients were 95.79 ± 51.40 and 128.85 ± 48.81 weeks, respectively. The 1-year PFS and OS were 60% and 100%, respectively, among our patients, and two patients had more than 3 years of OS and more than 2 years of PFS. It seems that intracerebral administration of bone marrow MSC containing the HSV-TK gene in combination with intravenous ganciclovir would be safe and feasible in the treatment of patients with GBM.






Similar content being viewed by others
Data Availability
Researchers could submit their research proposals to the corresponding author to access datasets of this clinical trial.
References
Garcia CR, Slone SA, Morgan RM et al (2018) Dose-dense temozolomide for recurrent high-grade gliomas: a single-center retrospective study. Med Oncol 35(10):136
Xie Q, Mittal S, Berens ME (2014) Targeting adaptive glioblastoma: an overview of proliferation and invasion. Neuro Oncol 16(12):1575–1584
Stupp R, Mason WP, Van Den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Westphal M, Ylä-Herttuala S, Martin J et al (2013) Adenovirus-mediated gene therapy with sitimageneceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 14(9):823–833
Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO (2016) Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson’s disease, glioma, and schwannoma. Cell Mol Neurobiol 36(3):417–427
Dixit K, Kumthekar P (2017) Gene delivery in neuro-oncology. Curr Oncol Reports 19(11):69
Choi SA, Lee JY, Wang KC et al (2012) Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur J Cancer 48(1):129–137
Fei S, Qi X, Kedong S, Guangchun J, Jian L, Wei Q (2012) The antitumor effect of mesenchymal stem cells transduced with a lentiviral vector expressing cytosine deaminase in a rat glioma model. J Cancer Res Clin Oncol 138(2):347–357
Namba H, Kawaji H, Yamasaki T (2016) Use of genetically engineered stem cells for glioma therapy (review). Oncol Lett 11(1):9–15
Nowakowski A, Andrzejewska A, Janowski M, Walczak P, Lukomska B (2013) Genetic engineering of stem cells for enhanced therapy. Acta Neurobiol Exp (Wars) 73(1):1–18
Naldini L, Blömer U, Gallay P et al (1996) In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272(5259):263–267
McGinley L, McMahon J, Strappe P et al (2011) Lentiviral vector mediated modification of mesenchymal stem cells & enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther 2(2):1–18
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36(4):568–584
Mahmood A, Lu D, Lu M et al (2003) Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery 53(3):697–703
Studeny M, Marini FC, Dembinski JL et al (2004) Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96(21):1593–1603
Rath P, Shi H, Maruniak JA, Litofsky NS, Maria BL, Kirk MD (2009) Stem cells as vectors to deliver HSV/tk gene therapy for malignant gliomas. Curr Stem Cell Res Ther 4(1):44–49
Hoyos V, Del Bufalo F, Yagyu S et al (2015) Mesenchymal stromal cells for linked delivery of oncolytic and apoptotic adenoviruses to non-small-cell lung cancers. Mol Ther 23(9):1497–1506
O’Brien KP, Khan S, Gilligan KE et al (2018) Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 37(16):2137–2149
Nakamura K, Ito Y, Kawano Y et al (2004) Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther 11(14):1155–1164
Yazdani SO, Hafizi M, Zali A-R et al (2013) Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy 15(7):782–791
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Klatzmann D, Valéry CA, Bensimon G et al (1998) A phase I/II study of herpes simplex virus type 1 thymidine kinase ‘suicide’ gene therapy for recurrent glioblastoma. Hum Gene Ther 9(17):2595–2604
Rainov NG (2000) A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastomamultiforme. Hum Gene Ther 11(17):2389–2401
Shand N, Weber F, Mariani L et al (1999) A phase 1–2 clinical trial of gene therapy for recurrent glioblastomamultiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. Hum Gene Ther 10(14):2325–2335
Ram Z, Culver KW, Oshiro EM et al (1997) Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med 3(12):1354–1361
Oldfield EH, Ram Z, Culver KW, Blaese RM, DeVroom HL, Anderson WF (1993) Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. National Institutes of Health. Human Gene Ther 4(1):39–69
Immonen A, Vapalahti M, Tyynela K et al (2004) AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther 10(5):967–972
Ji N, Weng D, Liu C et al (2016) Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 7(4):4369–4378
Pellinen R, Hakkarainen T, Wahlfors T et al (2004) Cancer cells as targets for lentivirus-mediated gene transfer and gene therapy. Int J Oncol 25(6):1753–1762
Metz MZ, Gutova M, Lacey SF et al (2013) Neural stem cell-mediated delivery of irinotecan-activating carboxylesterases to glioma: implications for clinical use. Stem Cells Transl Med 2(12):983–992
Kim SK, Kim SU, Park IH et al (2006) Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res 12(18):5550–5556
Grisendi G, Bussolari R, Cafarelli L et al (2010) Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res 70(9):3718–3729
Yin J, Kim JK, Moon JH et al (2011) hMSC-mediated concurrent delivery of endostatin and carboxylesterase to mouse xenografts suppresses glioma initiation and recurrence. Mol Ther 19(6):1161–1169
von Einem JC, Peter S, Günther C et al (2017) Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells-TREAT-ME-1-a phase I, first in human, first in class trial. Oncotarget 8(46):80156–80166.
von Einem JC, Guenther C, Volk H-D et al (2019) Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: results from the phase 1/2 TREAT-ME-1 trial. Int J Cancer 145(6):1538–1546
Pacioni S, D’Alessandris QG, Giannetti S et al (2017) Human mesenchymal stromal cells inhibit tumor growth in orthotopicglioblastomaxenografts. Stem Cell Res Ther 8(1):53–53
Altaner C, Altanerova V, Cihova M et al (2014) Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer 134(6):1458–1465
Kosaka H, Ichikawa T, Kurozumi K et al (2012) Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther 19(8):572–578
Ryu CH, Park KY, Kim SM et al (2012) Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem Biophys Res Commun 421(3):585–590
Wills KN, Huang W-M, Harris MP, Machemer T, Maneval DC, Gregory RJ (1995) Gene therapy for hepatocellular carcinoma: chemosensitivity conferred by adenovirus-mediated transfer of the HSV-1 thymidine kinase gene. Cancer Gene Ther 2(3):191–197
Ido A, Nakata K, Kato Y et al (1995) Gene therapy for hepatoma cells using a retrovirus vector carrying herpes simplex virus thymidine kinase gene under the control of human α-fetoprotein gene promoter. Can Res 55(14):3105–3109
Kanai F, Shiratori Y, Yoshida Y et al (1996) Gene therapy for α-fetoprotein-producing human hepatoma cells by adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene. Hepatology 23(6):1359–1368
Garver R Jr, Goldsmith KT, Rodu B, Hu PC, Sorscher EJ, Curiel DT (1994) Strategy for achieving selective killing of carcinomas. Gene Ther 1(1):46–50
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Shohada Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, for their support and assistance with this research.
Author information
Authors and Affiliations
Contributions
S.O.Y., M.H.A.P., and M.G. prepared the original draft; S.O.Y., A.R.Z., and M.S. finalized all drafts and approved the final version of the manuscript; S.O.Y. and A.R.Z. conceptualized the study and provided supervision; S.O.Y., M.H.A.P., M.G., G.S., F.R., and F.J.A. were involved in conducting the trial; all other authors were involved in data gathering; all authors reviewed and revised the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
This study was designed in line with the declaration of Helsinki and was approved by the Ethics committee in the Medical Research Committee of the Shahid Beheshti University of Medical Sciences; license number: IR.SBMU.REC.1396.224 and clinical trial registration number: IRCT20200502047277N2. All procedures were performed after written informed consent was obtained. Written consent included procedures of culture and analyses of biopsies from the tissue. Patients were fully aware of the experimental process of the treatment, unexpected outcomes, and possible or unforeseen adverse effects.
Consent for Publication
Patients have consented to the submission of the present study to the journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oraee-Yazdani, S., Akhlaghpasand, M., Shokri, G. et al. Intracerebral Administration of Autologous Mesenchymal Stem Cells as HSV-TK Gene Vehicle for Treatment of Glioblastoma Multiform: Safety and Feasibility Assessment. Mol Neurobiol 58, 4425–4436 (2021). https://doi.org/10.1007/s12035-021-02393-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02393-y