Abstract
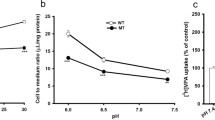
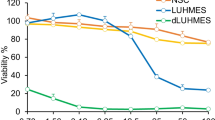
In Alzheimer’s disease (AD), excessive amounts of quinolinic acid (QUIN) accumulate within the brain parenchyma and dystrophic neurons. QUIN also regulates glutamate uptake into neurons, which may be due to modulation of Na+-dependent excitatory amino acid transporters (EAATs). To determine the biological relationships between QUIN and glutamate dysfunction, we first quantified the functionality and kinetics of [3H]QUIN uptake in primary human neurons using liquid scintillation. We then measured changes in the protein expression of the glutamate transporter EAAT3 and EAAT1b in primary neurons treated with QUIN and the EAAT inhibitor l-trans-pyrrolidine-2,4-dicarboxylic acid (2,4-PDC) using western blotting and immunohistochemistry. Immunohistochemistry was further used to elucidate intracellular transport of exogenous QUIN and the lysosomal-associated membrane protein 2 (LAMP2). Structural insights into the binding between QUIN and EAAT3 were further investigated using molecular docking techniques. We report significant temperature-dependent high-affinity transport leading to neuronal uptake of [3H]QUIN with a Km of 42.2 μM, and a Vmax of 9.492 pmol/2 min/mg protein, comparable with the uptake of glutamate. We also found that QUIN increases expression of the EAAT3 monomer while decreasing the functional trimer. QUIN uptake into primary neurons was shown to involve EAAT3 as uptake was significantly attenuated following EAAT inhibition. We also demonstrated that QUIN increases the expression of aberrant EAAT1b protein in neurons further implicating QUIN-induced glutamate dysfunction. Furthermore, we demonstrated that QUIN is metabolised exclusively in lysosomes. The involvement of EAAT3 as a modulator for QUIN uptake was further confirmed using molecular docking. This study is the first to characterise a mechanism for QUIN uptake into primary human neurons involving EAAT3, opening potential targets to attenuate QUIN-induced excitotoxicity in neuroinflammatory diseases.










Similar content being viewed by others
Abbreviations
- [3H]QUIN:
-
3H Quinolinic acid
- DAPI:
-
4′,6-Diamidino-2-phenylindole dihydrochloride
- ATP:
-
Adenosine triphosphate
- AD:
-
Alzheimer’s disease
- Aβ:
-
Amyloid beta
- ALS:
-
Amyotrophic lateral sclerosis
- Asp:
-
Aspartate
- CSF:
-
Cerebrospinal fluid
- MK-801:
-
Dizocilpine hydrogen maleate
- ER:
-
Endoplasmic reticulum
- EAAT:
-
Excitatory amino acid transporter
- FBS:
-
Foetal bovine serum
- GFAP:
-
Glial fibrillary acidic protein
- Glu:
-
Glutamate
- HBBS:
-
Hank’s balanced salt solution
- HD:
-
Huntington’s disease
- HCl:
-
Hydrochloric acid
- IDO-1:
-
Indoleamine 2,3 dioxygenase
- IFN-γ:
-
Interferon gamma
- KP:
-
Kynurenine pathway
- 2,4-PDC:
-
l-trans-pyrrolidine-2,4-dicarboxylic acid
- MAP 2:
-
Microtubule assembly protein 2
- NMDA-R:
-
N-methyl d-aspartate receptor
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NO:
-
Nitric oxide
- NGS:
-
Normal goat’s serum
- PBS:
-
Phosphate-buffered saline
- PKC:
-
Protein kinase C
- QPRTase:
-
Quinolinate phosphoribosyl transferase
- QUIN:
-
Quinolinic acid
- NaOH:
-
Sodium hydroxide
- TRP:
-
Tryptophan
- TNF-α:
-
Tumour necrosis factor alpha
References
Stone TW (1993) Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev 45(3):309–379
Braidy N, Grant R, Adams S, Brew BJ, Guillemin GJ (2009) Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res 16(1):77–86
Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ (2009) Effects of kynurenine pathway metabolites on intracellular NAD synthesis and cell death in human primary astrocytes and neurons. Int J Tryptophan Res 2:61–69
Tasset I, Pérez-De La Cruz V, Elinos-Calderón D, Carrillo-Mora P, González-Herrera IG, Luna-López A, Konigsberg M, Pedraza-Chaverrí J et al (2010) Protective effect of tert-butylhydroquinone on the quinolinic-acid-induced toxicity in rat striatal slices: role of the Nrf2-antioxidant response element pathway. Neurosignals 18(1):24–31
Tavares RG, Tasca CI, Santos CES, Alves ĹB, Porciúncula LO, Emanuelli T, Souza DO (2002) Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int 40(7):621–627
Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ (2009) The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One 4(7):e6344
Braidy N, Brew BJ, Inestrosa NC, Chung R, Sachdev P, Guillemin GJ (2014) Changes in cathepsin D and Beclin-1 mRNA and protein expression by the excitotoxin quinolinic acid in human astrocytes and neurons. Metab Brain Dis 29(3):873–883
Achtyes E, Keaton SA, Smart LA, Burmeister AR, Heilman PL, Krzyzanowski S, Nagalla M, Guillemin GJ et al (2020) Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun 83:239–247
Brundin L, Sellgren CM, Lim CK, Grit J, Pålsson E, Landén M, Samuelsson M, Lundgren K et al (2016) An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry 6(8):e865
Chatterjee P, Goozee K, Lim CK, James I, Shen K, Jacobs KR, Sohrabi HR, Shah T et al (2018) Alterations in serum kynurenine pathway metabolites in individuals with high neocortical amyloid-beta load: a pilot study. Sci Rep 8(1):8008
Chatterjee P, Zetterberg H, Goozee K, Lim CK, Jacobs KR, Ashton NJ, Hye A, Pedrini S et al (2019) Plasma neurofilament light chain and amyloid-beta are associated with the kynurenine pathway metabolites in preclinical Alzheimer's disease. J Neuroinflammation 16(1):186
Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, Arredouani A, Marre M et al (2015) The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring) 23(10):2066–2074
Garcez ML, Jacobs KR, Guillemin GJ (2019) Microbiota alterations in Alzheimer's disease: involvement of the kynurenine pathway and inflammation. Neurotox Res 36(2):424–436
Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ (2007) Characterization of the kynurenine pathway in human neurons. J Neurosci 27(47):12884–12892
Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, Jacobs KR, Balzan R et al (2019) Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry
Lim CK, Bilgin A, Lovejoy DB, Tan V, Bustamante S, Taylor BV, Bessede A, Brew BJ et al (2017) Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci Rep 7:41473
Lim CK, Essa MM, de Paula Martins R, Lovejoy DB, Bilgin AA, Waly MI, al-Farsi YM, al-Sharbati M et al (2016) Altered kynurenine pathway metabolism in autism: implication for immune-induced glutamatergic activity. Autism Res 9(6):621–631
Sofia MA, Ciorba MA, Meckel K, Lim CK, Guillemin GJ, Weber CR, Bissonnette M, Pekow JR (2018) Tryptophan metabolism through the kynurenine pathway is associated with endoscopic inflammation in ulcerative colitis. Inflamm Bowel Dis 24(7):1471–1480
Sundaram G, Lim CK, Brew BJ, Guillemin GJ (2020) Kynurenine pathway modulation reverses the experimental autoimmune encephalomyelitis mouse disease progression. J Neuroinflammation 17(1):176
Vidal C, Li W, Santner-Nanan B, Lim CK, Guillemin GJ, Ball HJ, Hunt NH, Nanan R et al (2015) The kynurenine pathway of tryptophan degradation is activated during osteoblastogenesis. Stem Cells 33(1):111–121
Yan EB, Frugier T, Lim CK, Heng B, Sundaram G, Tan M, Rosenfeld JV, Walker DW et al (2015) Activation of the kynurenine pathway and increased production of the excitotoxin quinolinic acid following traumatic brain injury in humans. J Neuroinflammation 12:110
Kerr SJ, Armati PJ, Pemberton LA, Smythe G, Tattam B, Brew BJ (1997) Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages. Neurology 49(6):1671–1681
Guillemin GJ, Meininger V, Brew BJ (2005) Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis 2(3–4):166–176
Campesan S, Green EW, Breda C, Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP, Giorgini F (2011) The kynurenine pathway modulates neurodegeneration in a Drosophila model of Huntington's disease. Curr Biol 21(11):961–966
Sathyasaikumar KV, Stachowski EK, Amori L, Guidetti P, Muchowski PJ, Schwarcz R (2010) Dysfunctional kynurenine pathway metabolism in the R6/2 mouse model of Huntington's disease. J Neurochem 113(6):1416–1425
Beal MF, Matson WR, Swartz KJ, Gamache PH, Bird ED (1990) Kynurenine pathway measurements in Huntington's disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem 55(4):1327–1339
Wu W, Nicolazzo JA, Wen L, Chung R, Stankovic R, Bao SS, Lim CK, Brew BJ et al (2013) Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer's disease brain. PLoS One 8(4):e59749
Gong CY, Li Z, Wang HM, Liu J, Chen L, Zhang HW, Wang X, Yang J (2011) Targeting the kynurenine pathway as a potential strategy to prevent and treat Alzheimer's disease. Med Hypotheses 77(3):383–385
Plangar I et al (2011) Targeting the kynurenine pathway-related alterations in Alzheimer's disease: a future therapeutic strategy. J Alzheimers Dis 24(Suppl 2):199–209
Ting KK, Brew B, Guillemin G (2007) The involvement of astrocytes and kynurenine pathway in Alzheimer's disease. Neurotox Res 12(4):247–262
Guillemin GJ, Brew BJ (2002) Implications of the kynurenine pathway and quinolinic acid in Alzheimer's disease. Redox Rep 7(4):199–206
Lim CK, Brew BJ, Sundaram G, Guillemin GJ (2010) Understanding the roles of the kynurenine pathway in multiple sclerosis progression. Int J Tryptophan Res 3:157–167
Guillemin GJ, Kerr SJ, Pemberton LA, Smith DG, Smythe GA, Armati PJ, Brew BJ (2001) IFN-beta1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. J Interf Cytokine Res 21(12):1097–1101
McRae A, Dahlstrom A, Ling EA (1997) Microglial in neurodegenerative disorders: emphasis on Alzheimer's disease. Gerontology 43(1–2):95–108
Alberati-Giani D, Cesura AM (1998) Expression of the kynurenine enzymes in macrophages and microglial cells: regulation by immune modulators. Amino Acids 14(1–3):251–255
Guillemin GJ, Smythe GA, Veas LA, Takikawa O, Brew BJ (2003) A beta 1-42 induces production of quinolinic acid by human macrophages and microglia. Neuroreport 14(18):2311–2315
Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D (2000) Tryptophan degradation and immune activation in Alzheimer's disease. J Neural Transm (Vienna) 107(3):343–353
Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM (2005) Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer's disease hippocampus. Neuropathol Appl Neurobiol 31(4):395–404
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65(1):1–105
Montiel T, Camacho A, Estrada-Sánchez AM, Massieu L (2005) Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience 133(3):667–678
Koch HP, Larsson HP (2005) Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters. J Neurosci 25(7):1730–1736
Storck T, Schulte S, Hofmann K, Stoffel W (1992) Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A 89(22):10955–10959
Pines G, Danbolt NC, Bjørås M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J et al (1992) Cloning and expression of a rat brain L-glutamate transporter. Nature 360(6403):464–467
Kanai Y, Hediger MA (1992) Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 360(6403):467–471
Fairman WA, Vandenberg RJ, Arriza JL, Kavanaught MP, Amara SG (1995) An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature 375(6532):599–603
Arriza JL, Eliasof S, Kavanaugh MP, Amara SG (1997) Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci U S A 94(8):4155–4160
Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW (1994) Localization of neuronal and glial glutamate transporters. Neuron 13(3):713–725
Huang YH, Dykes-Hoberg M, Tanaka K, Rothstein JD, Bergles DE (2004) Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J Neurosci 24(1):103–111
Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, Yawata I, Li H et al (2008) Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res 1210:11–19
Pow DV, Cook DG (2009) Neuronal expression of splice variants of "glial" glutamate transporters in brains afflicted by Alzheimer's disease: unmasking an intrinsic neuronal property. Neurochem Res 34(10):1748–1757
Gonzalez MI, Kazanietz MG, Robinson MB (2002) Regulation of the neuronal glutamate transporter excitatory amino acid carrier-1 (EAAC1) by different protein kinase C subtypes. Mol Pharmacol 62(4):901–910
Hu S, Sheng WS, Ehrlich LC, Peterson PK, Chao CC (2000) Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7(3):153–159
Sheldon AL, Robinson MB (2007) The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 51(6–7):333–355
Fournier KM, Gonzalez MI, Robinson MB (2004) Rapid trafficking of the neuronal glutamate transporter, EAAC1: evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. J Biol Chem 279(33):34505–34513
Trotti D, Rolfs A, Danbolt NC, Brown RH Jr, Hediger MA (1999) SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci 2(9):848
Martin-Hernandez D et al (2019) Chronic mild stress alters kynurenine pathways changing the glutamate neurotransmission in frontal cortex of rats. Mol Neurobiol 56(1):490–501
Guillemin GJ, Smith DG, Kerr SJ, Smythe GA, Kapoor V, Armati PJ, Brew BJ (2000) Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep 5(2–3):108–111
Foster AC, Miller LP, Oldendorf WH, Schwarcz R (1984) Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp Neurol 84(2):428–440
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 53:452–458
Heinzelmann G, Kuyucak S (2014) Molecular dynamics simulations of the mammalian glutamate transporter EAAT3. PLoS One 9(3):e92089
Werling LL, Nadler JV (1982) Complex binding of L-[3H]glutamate to hippocampal synaptic membranes in the absence of sodium. J Neurochem 38(4):1050–1062
Sharif NA, Roberts PJ (1981) L-Aspartate binding sites in rat cerebellum: a comparison of the binding of L-[3H]aspartate and L-[3H]glutamate to synaptic membranes. Brain Res 211(2):293–303
Henthorn TK, Liu Y, Mahapatro M, Ng KY (1999) Active transport of fentanyl by the blood-brain barrier. J Pharmacol Exp Ther 289(2):1084–1089
Dowd LA, Coyle AJ, Rothstein JD, Pritchett DB, Robinson MB (1996) Comparison of Na+-dependent glutamate transport activity in synaptosomes, C6 glioma, and Xenopus oocytes expressing excitatory amino acid carrier 1 (EAAC1). Mol Pharmacol 49(3):465–473
Velaz-Faircloth M, McGraw TS, alandro MS, Fremeau RT Jr, Kilberg MS, Anderson KJ (1996) Characterization and distribution of the neuronal glutamate transporter EAAC1 in rat brain. Am J Phys 270(1 Pt 1):C67–C75
Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG (1994) Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci 14(9):5559–5569
Bridges RJ, Kavanaugh MP, Chamberlin AR (1999) A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr Pharm Des 5(5):363–379
Bridges RJ, Lovering FE, Koch H, Cotman CW, Chamberlin AR (1994) A conformationally constrained competitive inhibitor of the sodium-dependent glutamate transporter in forebrain synaptosomes: L-anti-endo-3,4-methanopyrrolidine dicarboxylate. Neurosci Lett 174(2):193–197
Bridges RJ, Stanley MS, Anderson MW, Cotman CW, Chamberlin AR (1991) Conformationally defined neurotransmitter analogues. Selective inhibition of glutamate uptake by one pyrrolidine-2,4-dicarboxylate diastereomer. J Med Chem 34(2):717–725
Anderson CM et al (2001) Differing effects of substrate and non-substrate transport inhibitors on glutamate uptake reversal. J Neurochem 79(6):1207–1216
Blitzblau R, Gupta S, Djali S, Robinson MB, Rosenberg PA (1996) The glutamate transport inhibitor L-trans-pyrrolidine-2,4-dicarboxylate indirectly evokes NMDA receptor mediated neurotoxicity in rat cortical cultures. Eur J Neurosci 8(9):1840–1852
Bridges RJ, Natale NR, Patel SA (2012) System xc(−) cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165(1):20–34
Belousov AB, Fontes JD (2016) Role of neuronal gap junctions in NMDA receptor-mediated excitotoxicity and ischemic neuronal death. Neural Regen Res 11(1):75–76
Weilinger NL, Lohman AW, Rakai BD, Ma EMM, Bialecki J, Maslieieva V, Rilea T, Bandet MV et al (2016) Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci 19(3):432–442
Fischer W, Neubert RH, Brandsch M (2007) Clonidine accumulation in human neuronal cells. Eur J Pharm Sci 32(4–5):291–295
Kramer K, Baudry M (1984) Low concentrations of potassium inhibit the Na-dependent [3H]glutamate binding to rat hippocampal membranes. Eur J Pharmacol 102(1):155–158
Sarantis M, Attwell D (1990) Glutamate uptake in mammalian retinal glia is voltage- and potassium-dependent. Brain Res 516(2):322–325
Tanaka C, Nishizuka Y (1994) The protein kinase C family for neuronal signaling. Annu Rev Neurosci 17:551–567
Pierozan P, Zamoner A, Krombauer Soska Â, Bristot Silvestrin R, Oliveira Loureiro S, Heimfarth L, Mello e Souza T, Wajner M et al (2010) Acute intrastriatal administration of quinolinic acid provokes hyperphosphorylation of cytoskeletal intermediate filament proteins in astrocytes and neurons of rats. Exp Neurol 224(1):188–196
Gonzalez MI et al (2007) Constitutive endocytosis and recycling of the neuronal glutamate transporter, excitatory amino acid carrier 1. J Neurochem 103(5):1917–1931
Do SH, Kamatchi GL, Washington JM, Zuo Z (2002) Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3: the role of protein kinase C. Anesthesiology 96(6):1492–1497
Ye ZC, Sontheimer H (1996) Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport 7(13):2181–2185
Ting KK, Brew BJ, Guillemin GJ (2009) Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer's disease. J Neuroinflammation 6:36
Sullivan SM, Lee A, Björkman ST, Miller SM, Sullivan RKP, Poronnik P, Colditz PB, Pow DV (2007) Cytoskeletal anchoring of GLAST determines susceptibility to brain damage: an identified role for GFAP. J Biol Chem 282(40):29414–29423
Gonzalez MI, Robinson MB (2004) Protein kinase C-dependent remodeling of glutamate transporter function. Mol Interv 4(1):48–58
Thai DR (2002) Excitatory amino acid transporter EAAT-2 in tangle-bearing neurons in Alzheimer's disease. Brain Pathol 12(4):405–411
Vallejo-Illarramendi A, Domercq M, Matute C (2005) A novel alternative splicing form of excitatory amino acid transporter 1 is a negative regulator of glutamate uptake. J Neurochem 95(2):341–348
Macnab LT, Pow DV (2007) Central nervous system expression of the exon 9 skipping form of the glutamate transporter GLAST. Neuroreport 18(8):741–745
Shin JW, Nguyen KTD, Pow DV, Knight T, Buljan V, Bennett MR, Balcar VJ (2009) Distribution of glutamate transporter GLAST in membranes of cultured astrocytes in the presence of glutamate transport substrates and ATP. Neurochem Res 34(10):1758–1766
Scott HL, Pow DV, Tannenberg AEG, Dodd PR (2002) Aberrant expression of the glutamate transporter excitatory amino acid transporter 1 (EAAT1) in Alzheimer's disease. J Neurosci 22(3):RC206
Selkirk JV, Stiefel TH, Stone IM, Naeve GS, Foster AC, Poulsen DJ (2005) Over-expression of the human EAAT2 glutamate transporter within neurons of mouse organotypic hippocampal slice cultures leads to increased vulnerability of CA1 pyramidal cells. Eur J Neurosci 21(8):2291–2296
Kohler C, Eriksson LG, Flood PR, Hardie JA, Okuno E, Schwarcz R (1988) Quinolinic acid metabolism in the rat brain. Immunohistochemical identification of 3-hydroxyanthranilic acid oxygenase and quinolinic acid phosphoribosyltransferase in the hippocampal region. J Neurosci 8(3):975–987
Dunn WA Jr (1994) Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends Cell Biol 4(4):139–143
Malik AR, Willnow TE (2019) Excitatory amino acid transporters in physiology and disorders of the central nervous system. Int J Mol Sci:20(22)
Hoshi A, Tsunoda A, Yamamoto T, Tada M, Kakita A, Ugawa Y (2018) Altered expression of glutamate transporter-1 and water channel protein aquaporin-4 in human temporal cortex with Alzheimer's disease. Neuropathol Appl Neurobiol 44(6):628–638
Kobayashi E, Nakano M, Kubota K, Himuro N, Mizoguchi S, Chikenji T, Otani M, Mizue Y et al (2018) Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep 8(1):1712
Cassano T et al (2012) Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging 33(6):1121 e1–1121 12
Schallier A, Smolders I, van Dam D, Loyens E, de Deyn PP, Michotte A, Michotte Y, Massie A (2011) Region- and age-specific changes in glutamate transport in the AbetaPP23 mouse model for Alzheimer's disease. J Alzheimers Dis 24(2):287–300
Zumkehr J, Rodriguez-Ortiz CJ, Cheng D, Kieu Z, Wai T, Hawkins C, Kilian J, Lim SL et al (2015) Ceftriaxone ameliorates tau pathology and cognitive decline via restoration of glial glutamate transporter in a mouse model of Alzheimer's disease. Neurobiol Aging 36(7):2260–2271
Huang S, Tong H, Lei M, Zhou M, Guo W, Li G, Tang X, Li Z et al (2018) Astrocytic glutamatergic transporters are involved in Abeta-induced synaptic dysfunction. Brain Res 1678:129–137
Scimemi A, Meabon JS, Woltjer RL, Sullivan JM, Diamond JS, Cook DG (2013) Amyloid-beta1-42 slows clearance of synaptically released glutamate by mislocalizing astrocytic GLT-1. J Neurosci 33(12):5312–5318
Mookherjee P, Green PS, Watson GS, Marques MA, Tanaka K, Meeker KD, Meabon JS, Li N et al (2011) GLT-1 loss accelerates cognitive deficit onset in an Alzheimer's disease animal model. J Alzheimers Dis 26(3):447–455
Zoltowska KM, Maesako M, Meier J, Berezovska O (2018) Novel interaction between Alzheimer's disease-related protein presenilin 1 and glutamate transporter 1. Sci Rep 8(1):8718
Duerson K, Woltjer RL, Mookherjee P, Leverenz JB, Montine TJ, Bird TD, Pow DV, Rauen T et al (2009) Detergent-insoluble EAAC1/EAAT3 aberrantly accumulates in hippocampal neurons of Alzheimer's disease patients. Brain Pathol 19(2):267–278
Malik AR, Szydlowska K, Nizinska K, Asaro A, van Vliet EA, Popp O, Dittmar G, Fritsche-Guenther R et al (2019) SorCS2 controls functional expression of amino acid transporter EAAT3 and protects neurons from oxidative stress and epilepsy-induced pathology. Cell Rep 26(10):2792–2804 e6
Abrahamsen B, Schneider N, Erichsen MN, Huynh THV, Fahlke C, Bunch L, Jensen AA (2013) Allosteric modulation of an excitatory amino acid transporter: The subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. J Neurosci 33(3):1068–1087
Esslinger CS, Agarwal S, Gerdes J, Wilson PA, Davis ES, Awes AN, O'Brien E, Mavencamp T et al (2005) The substituted aspartate analogue L-beta-threo-benzyl-aspartate preferentially inhibits the neuronal excitatory amino acid transporter EAAT3. Neuropharmacology 49(6):850–861
Dunlop J (2006) Glutamate-based therapeutic approaches: Targeting the glutamate transport system. Curr Opin Pharmacol 6(1):103–107
Acknowledgements
The authors acknowledge the Curran Foundation (Australia) and the Rebecca Cooper foundation (Australia) for their philanthropic support.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This work has also been supported by the National Health and Medical Research Council (APP1128849). Dr. Nady Braidy is the recipient of an Australian Research Council Discovery Early Career Research Award (DE170100628) at the University of New South Wales.
Author information
Authors and Affiliations
Contributions
NB and HA performed the cell culture and molecular biology experiments. Molecular modelling was performed by JS. Experimental work was supervised by DP, JAN, GJG, and BJB. Figure 7 was drawn by B-EJ. All authors contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All authors confirm that all experiments were performed in accordance with relevant guidelines and regulations. Human foetal tissue was obtained following informed written consent. This has been approved by the Human Ethics Committees from the University of New South Wales (UNSW Ethic approval HREC 03187) and the Macquarie University HREC committee.
Consent for Publication
Not applicable
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Braidy, N., Alicajic, H., Pow, D. et al. Potential Mechanism of Cellular Uptake of the Excitotoxin Quinolinic Acid in Primary Human Neurons. Mol Neurobiol 58, 34–54 (2021). https://doi.org/10.1007/s12035-020-02046-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02046-6