Abstract
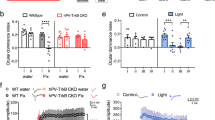
Perineuronal nets (PNNs) are condensed structures in the extracellular matrix that mainly surround GABA-ergic parvalbumin-positive interneurons in the adult brain. Previous studies revealed a parallel between PNN formation and the closure of the critical period. Moreover, ocular dominance plasticity is enhanced in response to PNN manipulations in adult animals. However, the mechanisms through which perineuronal nets modulate plasticity are still poorly understood. Recent work indicated that perineuronal nets may convey molecular signals by binding and storing proteins with important roles in cellular communication. Here we report that semaphorin3A (Sema3A), a chemorepulsive axon guidance cue known to bind to important perineuronal net components, is necessary to dampen ocular dominance plasticity in adult rats. First, we showed that the accumulation of Sema3A in PNNs in the visual cortex correlates with critical period closure, following the same time course of perineuronal nets maturation. Second, the accumulation of Sema3A in perineuronal nets was significantly reduced by rearing animals in the dark in the absence of any visual experience. Finally, we developed and characterized a tool to interfere with Sema3A signaling by means of AAV-mediated expression of receptor bodies, soluble proteins formed by the extracellular domain of the endogenous Sema3A receptor (neuropilin1) fused to a human IgG Fc fragment. By using this tool to antagonize Sema3A signaling in the adult rat visual cortex, we found that the specific inhibition of Sema3A promoted ocular dominance plasticity. Thus, Sema3A accumulates in perineuronal nets in an experience-dependent manner and its presence in the mature visual cortex inhibits plasticity.




Similar content being viewed by others
References
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251
Härtig W, Brauer K, Brückner G (1992) Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport 3:869–872
Carulli D, Rhodes KE, Fawcett JW (2007) Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol 501:83–94
Bukalo O, Schachner M, Dityatev A (2001) Modification of extracellular matrix by enzymatic removal of chondroitin sulfate and by lack of tenascin-R differentially affects several forms of synaptic plasticity in the hippocampus. Neuroscience 104:359–369
Ye Q, Miao Q-L (2013) Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol 32:352–363
Beurdeley M, Spatazza J, Lee HHC, Sugiyama S, Bernard C, Di Nardo AA et al (2012) Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci 32:9429–9437
Gogolla N, Caroni P, Luthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261
Lensjø KK, Lepperød ME, Dick G, Hafting T, Fyhn M (2017) Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci 37:1269–1283
Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L (2006) Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A 103:8517–8522
Sur M, Frost DO, Hockfield S (1988) Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci 8:874–882
Guimarães A, Zaremba S, Hockfield S (1990) Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci 10:3014–3024
Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R et al (2007) Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci 10:679–681
de Winter F, Kwok JCF, Fawcett JW, Vo TT, Carulli D, Verhaagen J (2016) The chemorepulsive protein semaphorin 3A and perineuronal net-mediated plasticity. Neural Plast 2016:3679545
Lensjø KK, Christensen AC, Tennøe S, Fyhn M, Hafting T (2017) Differential expression and cell-type specificity of perineuronal nets in hippocampus, medial entorhinal cortex, and visual cortex examined in the rat and mouse. eNeuro 4:ENEURO.0379–ENEU16.2017. https://doi.org/10.1523/ENEURO.0379-16.2017
Gu Y, Tran T, Murase S, Borrell A, Kirkwood A, Quinlan EM (2016) Neuregulin-dependent regulation of fast-spiking interneuron excitability controls the timing of the critical period. J Neurosci 36:10285–10295
Miao Q, Yao L, Rasch MJ, Ye Q, Li X, Zhang X (2016) Selective maturation of temporal dynamics of Intracortical excitatory transmission at the critical period onset. Cell Rep 16:1677–1689
He L-J, Liu N, Cheng T-L, Chen X-J, Li Y-D, Shu Y-S, Qiu ZL, Zhang XH (2014) Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat Commun 5:5036
Sun Y, Ikrar T, Davis MF, Gong N, Zheng X, Luo ZD, Lai C, Mei L et al (2016) Neuregulin-1/ErbB4 signaling regulates visual cortical plasticity. Neuron 92:160–173
Tang Y, Stryker MP, Alvarez-Buylla A, Espinosa JS (2014) Cortical plasticity induced by transplantation of embryonic somatostatin or parvalbumin interneurons. Proc Natl Acad Sci U S A 111:18339–18344
Morishita H, Cabungcal J-H, Chen Y, Do KQ, Hensch TK (2015) Prolonged period of cortical plasticity upon redox dysregulation in fast-spiking interneurons. Biol Psychiatry 78:396–402
Kobayashi Y, Ye Z, Hensch TK (2015) Clock genes control cortical critical period timing. Neuron 86:264–275
Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888
Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT (2013) A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501:543–546
Wang D, Fawcett J (2012) The perineuronal net and the control of CNS plasticity. Cell Tissue Res 349:147–160
Despras G, Bernard C, Perrot A, Cattiaux L, Prochiantz A, Lortat-Jacob H, Mallet JM (2013) Toward libraries of biotinylated chondroitin sulfate analogues: from synthesis to in vivo studies. Chemistry 19:531–540
Cardin AD, Weintraub HJ (1989) Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 9:21–32
De Wit J, De Winter F, Klooster J, Verhaagen J (2005) Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci 29:40–55
Dick G, Tan CL, Alves JN, Ehlert EME, Miller GM, Hsieh-Wilson LC, Sugahara K, Oosterhof A et al (2013) Semaphorin 3A binds to the perineuronal nets via chondroitin sulfate type E motifs in rodent brains. J Biol Chem 288:27384–27395
Vo T, Carulli D, Ehlert EME, Kwok JCF, Dick G, Mecollari V, Moloney EB, Neufeld G et al (2013) The chemorepulsive axon guidance protein semaphorin3A is a constituent of perineuronal nets in the adult rodent brain. Mol Cell Neurosci 56:186–200
Conrad AH, Zhang Y, Tasheva ES, Conrad GW (2010) Proteomic analysis of potential keratan sulfate, chondroitin sulfate A, and hyaluronic acid molecular interactions. Invest Ophthalmol Vis Sci 51:4500–4515
Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC (2011) VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell 22:2766–2776
Gu C, Limberg BJ, Whitaker GB, Perman B, Leahy DJ, Rosenbaum JS, Ginty DD, Kolodkin AL (2002) Characterization of neuropilin-1 structural features that confer binding to semaphorin 3A and vascular endothelial growth factor 165. J Biol Chem 277:18069–18076
Reed SE, Staley EM, Mayginnes JP, Pintel DJ, Tullis GE (2006) Transfection of mammalian cells using linear polyethylenimine is a simple and effective means of producing recombinant adeno-associated virus vectors. J Virol Methods 138:85–98
Kapfhammer JP, Xu H, Raper JA (2007) The detection and quantification of growth cone collapsing activities. Nat Protoc 2:2005–2011
Parker MW, Xu P, Li X, Vander Kooi CW (2012) Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J Biol Chem 287:11082–11089
Parker MW, Hellman LM, Xu P, Fried MG, Vander Kooi CW (2010) Furin processing of semaphorin 3F determines its anti-angiogenic activity by regulating direct binding and competition for neuropilin. Biochemistry 49:4068–4075
Parker MW, Vander Kooi CW (2017) Plate-based assay for measuring direct semaphorin-neuropilin interactions. Methods Mol Biol 1493:73–87
Hermens WT, ter Brake O, Dijkhuizen PA, Sonnemans MA, Grimm D, Kleinschmidt JA et al (1999) Purification of recombinant adeno-associated virus by iodixanol gradient ultracentrifugation allows rapid and reproducible preparation of vector stocks for gene transfer in the nervous system. Hum Gene Ther 10:1885–1891
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ et al (1999) Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6:973–985
Verhaagen J, Hobo B, Ehlert EME, Eggers R, Korecka JA, Hoyng SA et al (2018) Small scale production of recombinant adeno-associated viral vectors for gene delivery to the nervous system. Methods Mol Biol 1715:3–17
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. Gulf Professional Publishing
Nasarre C, Koncina E, Labourdette G, Cremel G, Roussel G, Aunis D, Bagnard D (2009) Neuropilin-2 acts as a modulator of Sema3A-dependent glioma cell migration. Cell Adhes Migr 3:383–389
Cariboni A, Davidson K, Rakic S, Maggi R, Parnavelas JG, Ruhrberg C (2011) Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Hum Mol Genet 20:336–344
Carulli D, Pizzorusso T, Kwok JCF, Putignano E, Poli A, Forostyak S, Andrews MR, Deepa SS et al (2010) Animals lacking link protein have attenuated perineuronal nets and persistent plasticity. Brain 133:2331–2347
Rowlands D, Lensjø KK, Dinh T, Yang S, Andrews MR, Hafting T, Fyhn M, Fawcett JW et al (2018) Aggrecan directs extracellular matrix-mediated neuronal plasticity. J Neurosci 38:10102–10113
Carcea I, Ma’ayan A, Mesias R, Sepulveda B, Salton SR, Benson DL (2010) Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A. J Neurosci 30:15317–15329
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J et al (2009) PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596
Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang XL, Bachoo R et al (2011) Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 31:14051–14066
Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG et al (2012) NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15:703–712
Carulli D, Foscarin S, Faralli A, Pajaj E, Rossi F (2013) Modulation of semaphorin3A in perineuronal nets during structural plasticity in the adult cerebellum. Mol Cell Neurosci 57:10–22
Wang Q, Chiu S-L, Koropouli E, Hong I, Mitchell S, Easwaran TP, Hamilton NR, Gustina AS et al (2017) Neuropilin-2/PlexinA3 receptors associate with GluA1 and mediate Sema3F-dependent homeostatic scaling in cortical neurons. Neuron 96:1084–1098.e7
Orr BO, Fetter RD, Davis GW (2017) Retrograde semaphorin-plexin signalling drives homeostatic synaptic plasticity. Nature 550:109–113
Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD et al (2009) Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature 462:1065–1069
Uesaka N, Uchigashima M, Mikuni T, Nakazawa T, Nakao H, Hirai H, Aiba A, Watanabe M et al (2014) Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science 344:1020–1023
Sahay A, Kim C-H, Sepkuty JP, Cho E, Huganir RL, Ginty DD et al (2005) Secreted semaphorins modulate synaptic transmission in the adult hippocampus. J Neurosci 25:3613–3620
Bouzioukh F, Daoudal G, Falk J, Debanne D, Rougon G, Castellani V (2006) Semaphorin3A regulates synaptic function of differentiated hippocampal neurons. Eur J Neurosci 23:2247–2254
Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M (1997) Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron 19:547–559
Takahashi T, Nakamura F, Jin Z, Kalb RG, Strittmatter SM (1998) Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci 1:487–493
Chen H, He Z, Bagri A, Tessier-Lavigne M (1998) Semaphorin–neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 21:1283–1290
Corredor M, Bonet R, Moure A, Domingo C, Bujons J, Alfonso I, Pérez Y, Messeguer À (2016) Cationic peptides and peptidomimetics bind glycosaminoglycans as potential Sema3A pathway inhibitors. Biophys J 110:1291–1303
Acknowledgments
The first four authors (marked with an asterisk) equally contributed to this study.
Funding
This work was supported by the EU 7th Framework program (FP7) Marie Curie actions (AxRegen) 2008–2012 to JV.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were carried out in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and were approved by the Italian Ministry of Health (authorization number 1152/2016-PR).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elena Maria Boggio, Erich M. Ehlert, Leonardo Lupori and Elizabeth B. Moloney contributed equally to this work.
Rights and permissions
About this article
Cite this article
Boggio, E.M., Ehlert, E.M., Lupori, L. et al. Inhibition of Semaphorin3A Promotes Ocular Dominance Plasticity in the Adult Rat Visual Cortex. Mol Neurobiol 56, 5987–5997 (2019). https://doi.org/10.1007/s12035-019-1499-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1499-0