Abstract
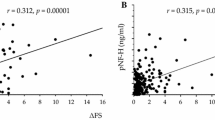
Amyotrophic lateral sclerosis (ALS) is a fatal motor neuron disease for which the existing candidate biomarkers (neurofilaments) have low specificity. Changes in blood IgG N-glycosylation have been observed in several diseases, including ALS, whereas cerebrospinal fluid (CSF) IgG has been less studied. Here, we characterized N-glycans of CSF IgG from ALS patients in comparison with a control group of other neurological diseases. Cerebrospinal fluid was collected from patients with ALS (n = 26) and other neurological diseases (n = 10). N-Glycans were released from CSF purified IgG with peptide N-glycosidase F, labeled with 2-aminobenzamide and analyzed by NP-HPLC chromatography in combination with exoglycosidase digestion and MALDI-TOF mass spectrometry. The N-glycosylation profile of ALS CSF IgG consisted of diantennary N-glycans predominantly with proximal fucose and some bisecting GlcNAc; agalacto-, mono-, and digalactosylated as well as α2,6-sialylated structures were detected. Differences between ALS and control patients were observed; most relevant was the increase in ALS CSF IgG of the level of galactosylated structures defined here as Gal-index (median 46.87 and 40.50% for ALS and controls, respectively; p = 0.006). The predictive value of the Gal-index (AUC = 0.792, p = 0.007) considering ROC analysis had potential utility as a diagnostic test for ALS and was comparable to that of phosphoneurofilament heavy chain (AUC = 0.777, p = 0.011), which was used as benchmark marker for our group of patients. The results provide the basis to further explore the potential of IgG N-glycan galactosylation as biomarker for ALS by using larger cohorts of patients and controls.



Similar content being viewed by others
References
Hardiman O, Al-Chalabi A, Brayne C, Beghi E, van den Berg LH, Chio A, Martin S et al (2017) The changing picture of amyotrophic lateral sclerosis: lessons from European registers. J Neurol Neurosurg Psychiatry 88:557–563. https://doi.org/10.1136/jnnp-2016-314495
Gouveia LO, de Carvalho M (2007) Young-onset sporadic amyotrophic lateral sclerosis: a distinct nosological entity? Amyotroph Lateral Scler 8:323–327. https://doi.org/10.1080/17482960701553956
Costa J, Gomes C, de Carvalho M (2010) Diagnosis, pathogenesis and therapeutic targets in amyotrophic lateral sclerosis. CNS Neurol Disord Drug Targets 9:764–778
Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C et al (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–322. https://doi.org/10.1016/S1474-4422(13)70036-X
Kruger T, Lautenschlager J, Grosskreutz J, Rhode H (2013) Proteome analysis of body fluids for amyotrophic lateral sclerosis biomarker discovery. Proteomics Clin Appl 7:123–135. https://doi.org/10.1002/prca.201200067
Costa J, de Carvalho M (2016) Emerging molecular biomarker targets for amyotrophic lateral sclerosis. Clin Chim Acta 455:7–14. https://doi.org/10.1016/j.cca.2016.01.011
Lehnert S, Costa J, de Carvalho M, Kirby J, Kuzma-Kozakiewicz M, Morelli C, Robberecht W, Shaw P et al (2014) Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 15:344–350. https://doi.org/10.3109/21678421.2014.884592
Goncalves M, Tillack L, de Carvalho M, Pinto S, Conradt HS, Costa J (2015) Phosphoneurofilament heavy chain and N-glycomics from the cerebrospinal fluid in amyotrophic lateral sclerosis. Clin Chim Acta 438:342–349. https://doi.org/10.1016/j.cca.2014.09.011
Oeckl P, Jardel C, Salachas F, Lamari F, Andersen PM, Bowser R, de Carvalho M, Costa J et al (2016) Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotroph Lateral Scler Frontotemporal Degener 17:404–413. https://doi.org/10.3109/21678421.2016.1167913
Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, von Arnim CA et al (2016) Neurofilaments in the diagnosis of motoneuron diseases: A prospective study on 455 patients. J Neurol Neurosurg Psychiatry 87:12–20. https://doi.org/10.1136/jnnp-2015-311387
Edri-Brami M, Rosental B, Hayoun D, Welt M, Rosen H, Wirguin I, Nefussy B, Drory VE et al (2012) Glycans in sera of amyotrophic lateral sclerosis patients and their role in killing neuronal cells. PLoS One 7:e35772. https://doi.org/10.1371/journal.pone.0035772
Edri-Brami M, Sharoni H, Hayoun D, Skutelsky L, Nemirovsky A, Porgador A, Lichtenstein RG (2015) Development of stage-dependent glycans on the Fc domains of IgG antibodies of ALS animals. Exp Neurol 267:95–106. https://doi.org/10.1016/j.expneurol.2015.02.023
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA (2007) The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol 25:21–50. https://doi.org/10.1146/annurev.immunol.25.022106.141702
Thompson E J (2005) Proteins of the cerebrospinal fluid 2nd edition London: Elsevier :p.16.
Plomp R, Bondt A, de Haan N, Rombouts Y, Wuhrer M (2016) Recent advances in clinical glycoproteomics of immunoglobulins (Igs). Mol Cell Proteomics 15:2217–2228. https://doi.org/10.1074/mcp.O116.058503
Wuhrer M, Selman MH, McDonnell LA, Kumpfel T, Derfuss T, Khademi M, Olsson T et al (2015) Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflammation 12(235):235. https://doi.org/10.1186/s12974-015-0450-1
Knopf J, Magorivska I, Maler JM, Spitzer P, Bilyy R, Biermann MHC, Hychka K, Bondt A et al (2018) Low amounts of bisecting glycans characterize cerebrospinal fluid-borne IgG. J Neuroimmunol 320:19–24. https://doi.org/10.1016/j.jneuroim.2018.04.010
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron D (2000) El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR (2016) The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener 17:414–425. https://doi.org/10.3109/21678421.2016.1140786
Bigge JC, Patel TP, Bruce JA, Goulding PN, Charles SM, Parekh RB (1995) Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal Biochem 230:229–238. https://doi.org/10.1006/abio.1995.1468
Costa J, Gatermann M, Nimtz M, Kandzia S, Glatzel M, Conradt HS (2018) N-glycosylation of extracellular vesicles from HEK-293 and glioma cell lines. Anal Chem 90:7871–7879. https://doi.org/10.1021/acs.analchem.7b05455
Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM (2008) GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res 7:1650–1659. https://doi.org/10.1021/pr7008252
Goncalves M, De Carvalho M, Peixoto C, Alves P, Barreto C, Oliva A, Pinto S et al (2017) Phosphoneurofilament heavy chain and vascular endothelial growth factor as cerebrospinal fluid biomarkers for ALS. Amyotroph Lateral Scler Frontotemporal Degener 18:134–136. https://doi.org/10.1080/21678421.2016.1212894
Dall'Olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, Franceschi C (2013) N-glycomic biomarkers of biological aging and longevity: a link with inflammaging. Ageing Res Rev 12:685–698. https://doi.org/10.1016/j.arr.2012.02.002
Wong AH, Fukami Y, Sudo M, Kokubun N, Hamada S, Yuki N (2016) Sialylated IgG-Fc: A novel biomarker of chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 87:275–279. https://doi.org/10.1136/jnnp-2014-309964
Seeling M, Bruckner C, Nimmerjahn F (2017) Differential antibody glycosylation in autoimmunity: Sweet biomarker or modulator of disease activity? Nat Rev Rheumatol 13:621–630. https://doi.org/10.1038/nrrheum.2017.146
Reusch D, Tejada ML (2015) Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 25:1325–1334. https://doi.org/10.1093/glycob/cwv065
Sussmuth SD, Sperfeld AD, Ludolph AC, Tumani H (2010) Hypercapnia is a possible determinant of the function of the blood-cerebrospinal fluid barrier in amyotrophic lateral sclerosis. Neurochem Res 35:1071–1074. https://doi.org/10.1007/s11064-010-0156-9
Ticozzi N, Tiloca C, Mencacci NE, Morelli C, Doretti A, Rusconi D, Colombrita C, Sangalli D et al (2013) Oligoclonal bands in the cerebrospinal fluid of amyotrophic lateral sclerosis patients with disease-associated mutations. J Neurol 260:85–92. https://doi.org/10.1007/s00415-012-6589-0
Pagan JD, Kitaoka M, Anthony RM (2018) Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 172(564–577):e513. https://doi.org/10.1016/j.cell.2017.11.041
Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ et al (2012) Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med 18:1401–1406. https://doi.org/10.1038/nm.2862
Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T et al (1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316:452–457
Holland M, Yagi H, Takahashi N, Kato K, Savage CO, Goodall DM, Jefferis R (2006) Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta 1760:669–677. https://doi.org/10.1016/j.bbagen.2005.11.021
van de Bovenkamp FS, Hafkenscheid L, Rispens T, Rombouts Y (2016) The emerging importance of IgG Fab glycosylation in immunity. J Immunol 196:1435–1441. https://doi.org/10.4049/jimmunol.1502136
Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M, Deelder AM (2011) IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J Proteome Res 10:143–152. https://doi.org/10.1021/pr1004373
Decker Y, Schomburg R, Nemeth E, Vitkin A, Fousse M, Liu Y, Fassbender K (2016) Abnormal galactosylation of immunoglobulin G in cerebrospinal fluid of multiple sclerosis patients. Mult Scler 22:1794–1803. https://doi.org/10.1177/1352458516631036
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. https://doi.org/10.1126/science.aag2590
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934. https://doi.org/10.1016/j.cell.2010.02.016
McGeer PL, McGeer EG (2002) Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 26:459–470. https://doi.org/10.1002/mus.10191
Lall D, Baloh RH (2017) Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia. J Clin Invest 127:3250–3258. https://doi.org/10.1172/JCI90607
Russell AC, Simurina M, Garcia MT, Novokmet M, Wang Y, Rudan I, Campbell H et al (2017) The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson’s disease. Glycobiology 27:501–510. https://doi.org/10.1093/glycob/cwx022
Funding
This work was supported by the EU JPND project SOPHIA (JPND/0003/2011), Fundação para a Ciência e a Tecnologia (FCT); Portugal and Euronanomed 2 ERA-NET project GlioEx (ENMed/0001/2013), FCT, Portugal; iNOVA4Health Research Unit (LISBOA-01-0145-FEDER-007344), which is cofunded by FCT/Ministério da Ciência e do Ensino Superior, through national funds; and by FEDER under the PT2020 Partnership Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Patients signed permission for biobank storage, and further studies were agreed by the local Ethic’s committee. The research was done in accordance with the Helsinki Declaration as revised in 2013 (www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 3259 kb)
Rights and permissions
About this article
Cite this article
Costa, J., Streich, L., Pinto, S. et al. Exploring Cerebrospinal Fluid IgG N-Glycosylation as Potential Biomarker for Amyotrophic Lateral Sclerosis. Mol Neurobiol 56, 5729–5739 (2019). https://doi.org/10.1007/s12035-019-1482-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1482-9