Abstract
Oxygen and glucose (OGD) deprivation is one of the most important pathogenic mechanisms in cerebral infarction and is widely used as an in vitro model for ischemic stroke. OGD also damages neural stem cells (NSCs), which are important in brain recovery after cerebral infarction. To enhance recovery, there have been many studies aimed at determining methods to protect NSCs after stroke. Because atorvastatin has diverse protective effects on neural cells, we studied whether it could rejuvenate NSCs injured by OGD. Primary cultured NSCs were exposed to OGD for 8 h, and the main characteristics of stem cells, such as survival, proliferation, migration, and differentiation, were evaluated to confirm the effect of OGD on NSCs. Next, cells were treated with various concentrations of atorvastatin with exposure to OGD for 8 h to confirm whether it could rejuvenate NSCs. OGD significantly affected the survival, proliferation, migration, and differentiation of NSCs. However, treatment with atorvastatin meaningfully restored survival, proliferation, migration, and differentiation of NSCs. These beneficial effects of atorvastatin were blocked by treatment with either a PI3K inhibitor or an ERK inhibitor. In conclusion, OGD damages NSCs and causes them to lose the main characteristics of stem cells so that they cannot contribute to brain recovery after cerebral infarction. However, treatment with atorvastatin after cerebral infarction can effectively rejuvenate NSCs through activating the PI3K and ERK pathways to aid in brain regeneration.








Similar content being viewed by others
References
Le DA, Wu Y, Huang Z, Matsushita K, Plesnila N, Augustinack JC, Hyman BT, Yuan J et al (2002) Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A 99(23):15188–15193. https://doi.org/10.1073/pnas.232473399
Malagelada C, Xifro X, Minano A, Sabria J, Rodriguez-Alvarez J (2005) Contribution of caspase-mediated apoptosis to the cell death caused by oxygen-glucose deprivation in cortical cell cultures. Neurobiol Dis 20(1):27–37. https://doi.org/10.1016/j.nbd.2005.01.028
Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, collaborators S (2010) A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 28 (6):1099–1106. doi:https://doi.org/10.1002/stem.430
Lee SH, Choi NY, Yu HJ, Park J, Choi H, Lee KY, Huh YM, Lee YJ et al (2016) Atorvastatin protects NSC-34 motor neurons against oxidative stress by activating PI3K, ERK and free radical scavenging. Mol Neurobiol 53(1):695–705. https://doi.org/10.1007/s12035-014-9030-0
Chen J, Zacharek A, Li A, Cui X, Roberts C, Lu M, Chopp M (2008) Atorvastatin promotes presenilin-1 expression and Notch1 activity and increases neural progenitor cell proliferation after stroke. Stroke 39(1):220–226. https://doi.org/10.1161/STROKEAHA.107.490946
Jung KH, Chu K, Jeong SW, Han SY, Lee ST, Kim JY, Kim M, Roh JK (2004) HMG-CoA reductase inhibitor, atorvastatin, promotes sensorimotor recovery, suppressing acute inflammatory reaction after experimental intracerebral hemorrhage. Stroke 35(7):1744–1749. https://doi.org/10.1161/01.STR.0000131270.45822.85
Chojnacki A, Weiss S (2008) Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat Protoc 3(6):935–940. https://doi.org/10.1038/nprot.2008.55
Lee J, Park HH, KoH SH, Choi H (2017) Neural stem cell death mechanisms induced by amyloid beta. Dement Neurocogn Disord 16(4):121–127. https://doi.org/10.12779/dnd.2017.16.4.121
Studer L, Tabar V, McKay RD (1998) Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci 1(4):290–295. https://doi.org/10.1038/1105
Bak SW, Choi H, Park HH, Lee KY, Lee YJ, Yoon MY, Koh SH (2016) Neuroprotective effects of acetyl-L-carnitine against oxygen-glucose deprivation-induced neural stem cell death. Mol Neurobiol 53(10):6644–6652. https://doi.org/10.1007/s12035-015-9563-x
Li C, Issa R, Kumar P, Hampson IN, Lopez-Novoa JM, Bernabeu C, Kumar S (2003) CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 116(Pt 13):2677–2685. https://doi.org/10.1242/jcs.00470
Koh SH, Noh MY, Cho GW, Kim KS, Kim SH (2009) Erythropoietin increases the motility of human bone marrow-multipotent stromal cells (hBM-MSCs) and enhances the production of neurotrophic factors from hBM-MSCs. Stem Cells Dev 18(3):411–421. https://doi.org/10.1089/scd.2008.0040
Sorrells SF, Paredes MF, Cebrian-Silla A, Sandoval K, Qi D, Kelley KW, James D, Mayer S et al (2018) Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555(7696):377–381. https://doi.org/10.1038/nature25975
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702. https://doi.org/10.1016/j.neuron.2011.05.001
Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97(6):703–716. https://doi.org/10.1016/S0092-8674(00)80783-7
Robel S, Berninger B, Gotz M (2011) The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12(2):88–104. https://doi.org/10.1038/nrn2978
Jones HM, Fang Z, Sun W, Clark LH, Stine JE, Tran AQ, Sullivan SA, Gilliam TP et al (2017) Atorvastatin exhibits anti-tumorigenic and anti-metastatic effects in ovarian cancer in vitro. Am J Cancer Res 7(12):2478–2490
Oliveira KA, Dal-Cim T, Lopes FG, Ludka FK, Nedel CB, Tasca CI (2018) Atorvastatin promotes cytotoxicity and reduces migration and proliferation of human A172 glioma cells. Mol Neurobiol 55(2):1509–1523. https://doi.org/10.1007/s12035-017-0423-8
Schulz JG, Bosel J, Stoeckel M, Megow D, Dirnagl U, Endres M (2004) HMG-CoA reductase inhibition causes neurite loss by interfering with geranylgeranylpyrophosphate synthesis. J Neurochem 89(1):24–32. https://doi.org/10.1046/j.1471-4159.2003.02305.x
Koh SH, Lo EH (2015) The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J Clin Neurol 11(4):297–304. https://doi.org/10.3988/jcn.2015.11.4.29711.e29
Wu Y, Peng H, Cui M, Whitney NP, Huang Y, Zheng JC (2009) CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. J Neurochem 109(4):1157–1167. https://doi.org/10.1111/j.1471-4159.2009.06043.x
Takahashi K, Murakami M, Yamanaka S (2005) Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells. Biochem Soc Trans 33(Pt 6):1522–1525. https://doi.org/10.1042/BST20051522
Hossini AM, Quast AS, Plotz M, Grauel K, Exner T, Kuchler J, Stachelscheid H, Eberle J et al (2016) PI3K/AKT signaling pathway is essential for survival of induced pluripotent stem cells. PLoS One 11(5):e0154770. https://doi.org/10.1371/journal.pone.0154770
Li S, Deng L, Gong L, Bian H, Dai Y, Wang Y (2010) Upregulation of CXCR4 favoring neural-like cells migration via AKT activation. Neurosci Res 67(4):293–299. https://doi.org/10.1016/j.neures.2010.04.006
Mebratu Y, Tesfaigzi Y (2009) How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 8(8):1168–1175. https://doi.org/10.4161/cc.8.8.8147
Li Z, Theus MH, Wei L (2006) Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Develop Growth Differ 48(8):513–523. https://doi.org/10.1111/j.1440-169X.2006.00889.x
Rhee YH, Yi SH, Kim JY, Chang MY, Jo AY, Kim J, Park CH, Cho JY et al (2016) Neural stem cells secrete factors facilitating brain regeneration upon constitutive Raf-Erk activation. Sci Rep 6:32025. https://doi.org/10.1038/srep32025
De Filippis L, Binda E (2012) Concise review: self-renewal in the central nervous system: neural stem cells from embryo to adult. Stem Cells Transl Med 1(4):298–308. https://doi.org/10.5966/sctm.2011-0045
Solozobova V, Wyvekens N, Pruszak J (2012) Lessons from the embryonic neural stem cell niche for neural lineage differentiation of pluripotent stem cells. Stem Cell Rev 8(3):813–829. https://doi.org/10.1007/s12015-012-9381-8
Liu Q, Fan X, Zhu J, Xu G, Li Y, Liu X (2014) Co-culturing improves the OGD-injured neuron repairing and NSCs differentiation via Notch pathway activation. Neurosci Lett 559:1–6. https://doi.org/10.1016/j.neulet.2013.11.027
Funding
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea, which is funded by the Ministry of Science, ICT, and Future Planning (2018R1A2A2A15023219); by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C2160 and HI18C1254); by the Medical Research Center (2017R1A5A2015395); and by the Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Science, ICT, and Future Planning (2017R1C1B5076630).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were conducted in accordance with Hanyang University’s guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee (IACUC) of Hanyang University.
Electronic Supplementary Material
Supplementary Fig. 1
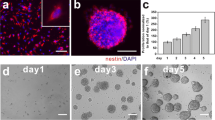
Difference of nestin expression depending on days after differentiation of neural stem cells. Nestin was still expressed in the cells for 9 days after induction of differentiation, but it decreased with time. To confirm whether nestin would disappear with differentiation of NSCs, additional experiments for 11-day differentiation were performed. In these additional experiments, it was confirmed that nestin expression almost disappeared 11 days after differentiation as DCX and GFAP expression increased, indicating complete differentiation of NSCs. (PNG 4052 kb)
Supplementary Fig. 2
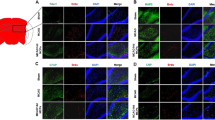
Effect of atorvastatin on the viability of neuronal stem cells (NSCs) after treatment with 16-h OGD and/or atorvastatin. Exposure to 16-h OGD reduced the viability of NSCs by about 60%, which was considered too low to be used for further experiments that require large amounts of cells, such as Western blotting. Atorvastatin significantly restored viability at concentrations of 0.1 and 1 μM. Data are presented as the mean (% of control) ± SD. Treatment groups were compared with the control group using two-way ANOVA followed by Tukey’s test (a: n=4, b: n=4). *P < 0.05, **P < 0.01 (vs. the control group); # P < 0.05, ## P < 0.01 (vs. the group that was treated with 16-h OGD alone) (PNG 107 kb)
Rights and permissions
About this article
Cite this article
Choi, NY., Kim, J.Y., Hwang, M. et al. Atorvastatin Rejuvenates Neural Stem Cells Injured by Oxygen–Glucose Deprivation and Induces Neuronal Differentiation Through Activating the PI3K/Akt and ERK Pathways. Mol Neurobiol 56, 2964–2977 (2019). https://doi.org/10.1007/s12035-018-1267-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1267-6