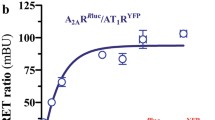
Abstract
While the role of the ascending dopaminergic system in brain function and dysfunction has been a subject of extensive research, the role of the descending dopaminergic system in spinal cord function and dysfunction is just beginning to be understood. Adenosine plays a key role in the inhibitory control of the ascending dopaminergic system, largely dependent on functional complexes of specific subtypes of adenosine and dopamine receptors. Combining a selective destabilizing peptide strategy with a proximity ligation assay and patch-clamp electrophysiology in slices from male mouse lumbar spinal cord, the present study demonstrates the existence of adenosine A1-dopamine D1 receptor heteromers in the spinal motoneuron by which adenosine tonically inhibits D1 receptor-mediated signaling. A1-D1 receptor heteromers play a significant control of the motoneuron excitability, represent main targets for the excitatory effects of caffeine in the spinal cord and can constitute new targets for the pharmacological therapy after spinal cord injury, motor aging-associated disorders and restless legs syndrome.





Similar content being viewed by others
References
McCarley RW (2007) Neurobiology of REM and NREM sleep. Sleep Med 8(4):302–330
Ferré S (2010) Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis 20(Suppl 1):S35–S49. https://doi.org/10.3233/JAD-2010-1400
Fredholm BB (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14(7):1315–1323
Ferré S (2016) Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology 233(10):1963–1979. https://doi.org/10.1007/s00213-016-4212-2
Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev 63(1):1–34. https://doi.org/10.1124/pr.110.003285
Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K (1997) Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci 20(10):482–487
Ferré S (2008) An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem 105(4):1067–1079
Navarro G, Aguinaga D, Moreno E, Hradsky J, Reddy PP, Cortés A, Mallol J, Casadó V et al (2014) Intracellular calcium levels determine differential modulation of allosteric interactions within G protein-coupled receptor heteromers. Chem Biol 21(11):1546–1556. https://doi.org/10.1016/j.chembiol.2014.10.004
Bonaventura J, Navarro G, Casadó-Anguera V, Azdad K, Rea W, Moreno E, Brugarolas M, Mallol J et al (2015) Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer. Proc Natl Acad Sci U S A 112(27):E3609–E3618. https://doi.org/10.1073/pnas.1507704112.
Ferré S, Popoli P, Giménez-Llort L, Finnman UB, Martínez E, Scotti de Carolis A, Fuxe K (1994) Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport 6(1):73–76
Ferré S, O’Connor WT, Svenningsson P, Bjorklund L, Lindberg J, Tinner B, Stromberg I, Goldstein M et al (1996) Dopamine D1 receptor-mediated facilitation of GABAergic neurotransmission in the rat strioentopenduncular pathway and its modulation by adenosine A1 receptor-mediated mechanisms. Eur J Neurosci 8(7):1545–1553
Ferré S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, Fredholm BB, Fuxe K (1998) Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J Biol Chem 273(8):4718–4724
Ferré S, Rimondini R, Popoli P, Reggio R, Pèzzola A, Hansson AC, Andersson A, Fuxe K (1999) Stimulation of adenosine A1 receptors attenuates dopamine D1 receptor-mediated increase of NGFI-A, c-fos and Jun-B mRNA levels in the dopamine-denervated striatum and dopamine D1 receptor-mediated turning behaviour. Eur J Neurosci 11(11):3884–3892
Popoli P, Giménez-Llort L, Pezzola A, Reggio R, Martínez E, Fuxe K, Ferré S (1996) Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neurosci Lett 218(3):209–213
Rimondini R, Ferré S, Giménez-Llort L, Ogren SO, Fuxe K (1998) Differential effects of selective adenosine A1 and A2A receptor agonists on dopamine receptor agonist-induced behavioural responses in rats. Eur J Pharmacol 347(2–3):153–158
Mayfield RD, Jones BA, Miller HA, Simosky JK, Larson GA, Zahniser NR (1999) Modulation of endogenous GABA release by an antagonistic adenosine A1/dopamineD1 receptor interaction in rat brain limbic regions but not basal ganglia. Synapse 33(4):274–281
Ginés S, Hillion J, Torvinen M, Le Crom S, Casadó V, Canela EI, Rondin S, Lew JY et al (2000) Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci U S A 97(15):8606–8611
Florán B, Barajas C, Florán L, Erlij D, Aceves J (2002) Adenosine A1 receptors control dopamine D1-dependent [(3)H]GABA release in slices of substantia nigra pars reticulata and motor behavior in the rat. Neuroscience 115(3):743–751
Le Crom S, Prou D, Vernier P (2002) Autocrine activation of adenosine A1 receptors blocks D1A but not D1B dopamine receptor desensitization. J Neurochem 82(6):1549–1552
Schwienbacher I, Fendt M, Hauber W, Koch M (2002) Dopamine D1 receptors and adenosine A1 receptors in the rat nucleus accumbens regulate motor activity but not prepulse inhibition. Eur J Pharmacol 444(3):161–169
Toda S, Alguacil LF, Kalivas PW (2003) Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J Neurochem 87(6):1478–1484
Batista LC, Prediger RD, Morato GS, Takahashi RN (2005) Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to ethanol in mice. Psychopharmacology 181(4):714–721
Cao Y, Xie KQ, Zhu XZ (2007) The enhancement of dopamine D1 receptor desensitization by adenosine A1 receptor activation. Eur J Pharmacol 562(1–2):34–38
Uustare A, Reinart R, Rinken A (2006) Modulation of dopamine D1 receptor signaling by adenosine A1 receptors in Sf9 cells requires expression of Gi proteins. Neurosci Lett 406(3):169–173
Yabuuchi K, Kuroiwa M, Shuto T, Sotogaku N, Snyder GL, Higashi H, Tanaka M, Greengard P et al (2006) Role of adenosine A1 receptors in the modulation of dopamine D1 and adenosine A2A receptor signaling in the neostriatum. Neuroscience 141(1):19–25
Sakiyama Y, Hatano K, Kato T, Tajima T, Kawasumi Y, Ito K (2007) Stimulation of adenosine A1 receptors decreases in vivo dopamine D1 receptor binding of [11C]SCH23390 in the cat striatum revealed by positron emission tomography. Ann Nucl Med 21(8):447–453
Hobson BD, O’Neill CE, Levis SC, Monteggia LM, Neve RL, Self DW, Bachtell RK (2013) Adenosine A1 and dopamine D1 receptor regulation of AMPA receptor phosphorylation and cocaine-seeking behavior. Neuropsychopharmacology 38(10):1974–1983. https://doi.org/10.1038/npp.2013.96
Shen J, Zhang L, Song WL, Meng T, Wang X, Chen L, Feng LY, Xu YC et al (2013) Design, synthesis and biological evaluation of bivalent ligands against a(1)-D(1) receptor heteromers. Acta Pharmacol Sin 34(3):441–452. https://doi.org/10.1038/aps.2012.151
Mango D, Bonito-Oliva A, Ledonne A, Cappellacci L, Petrelli R, Nisticò R, Berretta N, Fisone G et al (2014) Adenosine A1 receptor stimulation reduces D1 receptor-mediated GABAergic transmission from striato-nigral terminals and attenuates l-DOPA-induced dyskinesia in dopamine-denervated mice. Exp Neurol 261:733–743. https://doi.org/10.1016/j.expneurol.2014.08.022
Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP et al (2014) G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66(2):413–434. https://doi.org/10.1124/pr.113.008052
Gomes I, Ayoub MA, Fujita W, Jaeger WC, Pfleger KD, Devi LA (2016) G protein-coupled receptor heteromers. Annu Rev Pharmacol Toxicol 56:403–425. https://doi.org/10.1146/annurev-pharmtox-011613-135952
Acevedo J, Santana-Almansa A, Matos-Vergara N, Marrero-Cordero LR, Cabezas-Bou E, Díaz-Ríos M (2016) Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms. Neuropharmacology 101:490–505. https://doi.org/10.1016/j.neuropharm.2015.10.020
He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, Li Q, Yang H et al (2011) Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron 69:120–113. https://doi.org/10.1016/j.neuron.2010.12.001
Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sánchez-Soto M, Kumar-Barodia S, Naidu YT et al (2014) Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol 86:417–429. https://doi.org/10.1124/mol.114.093096
Navarro G, Cordomí A, Casadó-Anguera V, Moreno E, Cai N-S, Cortés A, Canela EI, Dessauer CW et al (2018) Evidence for functional pre-coupled complexes of receptor heteromers and adenylyl cyclase. Nat Commun 9:1242. https://doi.org/10.1038/s41467-018-03522-3
Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF (1999) In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 285:1569–1572
Zhong G, Díaz-Ríos M, Harris-Warrick RM (2006) Serotonin modulates the properties of ascending commissural interneurons in the neonatal mouse spinal cord. J Neurophysiol 95:1545–1555
Zhong G, Díaz-Ríos M, Harris-Warrick RM (2006) Intrinsic and functional differences among commissural interneurons during fictive locomotion and serotonergic modulation in the neonatal mouse. J Neurosci 26:6509–6517
Marullo S, Bouvier M (2007) Resonance energy transfer approaches in molecular pharmacology and beyond. Trends Pharmacol Sci 28:362–365
Ferré S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR et al (2009) Building a new conceptual framework for receptor heteromers. Nat Chem Biol 5:131–134. https://doi.org/10.1038/nchembio0309-131
Kjaerulff O, Barajon I, Kiehn O (1994) Sulphorhodamine-labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. J Physiol 478:265–273
Kjaerulff O, Kiehn O (1996) Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16:5777–5794
Kiehn O, Johnson BR, Raastad M (1996) Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience 75:263–273
Tresch MC, Kiehn O (1999) Coding of locomotor phase in populations of neurons in rostral and caudal segments of the neonatal rat lumbar spinal cord. J Neurophysiol 82:3563–3574
Butt SJ, Harris-Warrick RM, Kiehn O (2002) Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci 22:9961–9971
Butt SJ, Kiehn O (2003) Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron 38:953–963
Deuchars SA, Brooke RE, Deuchars J (2001) Adenosine A1 receptors reduce release from excitatory but not inhibitory synaptic inputs onto lateral horn neurons. J Neurosci 21:6308–6320
Zhu H, Clemens S, Sawchuk M, Hochman S (2007) Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience 149:885–897
Cassada DC, Tribble CG, Long SM, Kaza AK, Linden J, Rieger JM, Rosin D, Kron IL et al (2002) Adenosine A2A agonist reduces paralysis after spinal cord ischemia: correlation with A2A receptor expression on motor neurons. Ann Thorac Surg 74:846–849
Yokoyama C, Okamura H, Nakajima T, Taguchi J, Ibata Y (1994) Autoradiographic distribution of [3H]YM-09151-2, a high-affinity and selective antagonist ligand for the dopamine D2 receptor group, in the rat brain and spinal cord. J Comp Neurol 344:121–136
Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN (2009) Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology 34(4):972–986. https://doi.org/10.1038/npp.2008.144
Fernández-Dueñas V, Taura JJ, Cottet M, Gómez-Soler M, López-Cano M, Ledent C, Watanabe M, Trinquet E et al (2015) Untangling dopamine-adenosine receptor–receptor assembly in experimental parkinsonism in rats. Dis Model Mech 8:57–63. https://doi.org/10.1242/dmm.018143
Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG (2008) Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57:183–191
Clemens S, Belin-Rauscent A, Simmers J, Combes D (2012) Opposing modulatory effects of D1- and D2-like receptor activation on a spinal central pattern generator. J Neurophysiol 107:2250–2259. https://doi.org/10.1152/jn.00366.2011
Sharples SA, Koblinger K, Humphreys JM, Whelan PJ (2014) Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits 8:55. https://doi.org/10.3389/fncir.2014.00055
Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR (2005) Spinal cord transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci 25:11738–11747
Roy RR, Harkema SJ, Edgerton VR (2012) Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil 93:1487–1497. https://doi.org/10.1016/j.apmr.2012.04.034
Krucoff MO, Rahimpour S, Slutzky MW, Edgerton VR, Turner DA (2016) Enhancing nervous system recovery through neurobiologics, neural interface training, and neurorehabilitation. Front Neurosci 10:584. https://doi.org/10.3389/fnins.2016.00584
Miyazaki N, Nakatsuka T, Takeda D, Nohda K, Inoue K, Yoshida M (2008) Adenosine modulates excitatory synaptic transmission and suppresses neuronal death induced by ischaemia in rat spinal motoneurones. Pflugers Arch 457:441–451. https://doi.org/10.1007/s00424-008-0542-1
Witts EC, Panetta KM, Miles GB (2012) Glial-derived adenosine modulates spinal motor networks in mice. J Neurophysiol 107:1925–1934. https://doi.org/10.1152/jn.00513.2011
Witts EC, Nascimento F, Miles GB (2015) Adenosine-mediated modulation of ventral horn interneurons and spinal motoneurons in neonatal mice. J Neurophysiol 114:2305–2315. https://doi.org/10.1152/jn.00574.2014
Acton D, Miles GB (2015) Stimulation of glia reveals modulation of mammalian spinal motor networks by adenosine. PLoS One 10:e0134488. https://doi.org/10.1371/journal.pone.0134488
Allen EN, Cavanaugh JE (2014) Loss of motor coordination in an aging mouse model. Behav Brain Res 26:119–125. https://doi.org/10.1016/j.bbr.2014.03.032.
Cham R, Perera S, Studenski SA, Bohnen NI (2007) Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture 26:516–525
Keeler BE, Lallemand P, Patel MM, de Castro Brás LE, Clemens S (2016) Opposing aging-related shift of excitatory dopamine D1 and inhibitory D3 receptor protein expression in striatum and spinal cord. J Neurophysiol 115:363–369. https://doi.org/10.1152/jn.00390.2015
Quiroz C, Gulyani S, Ruiqian W, Bonaventura J, Cutler R, Pearson V, Allen RP, Earley CJ et al (2016) Adenosine receptors as markers of brain iron deficiency: implications for restless legs syndrome. Neuropharmacology 111:160–168. https://doi.org/10.1016/j.neuropharm.2016.09.002
Ferré S, Quiroz C, Guitart X, Rea W, Seyedian A, Moreno E, Casadó-Anguera V, Díaz-Ríos M et al (2018) Pivotal role of adenosine neurotransmission in restless legs syndrome. Front Neurosci 11:722. https://doi.org/10.3389/fnins.2017.00722
Acknowledgements
We would like to thank Dr. Thomas Cleland (Cornell University) for providing the scripts for electrophysiological data analysis, Joselyne Álvarez-González, Garrett Seale, Amelia Merced, and Andrea Husch for technical assistance.
Funding
This work was supported by COBRE Center for Neuroplasticity (NIH NIGMS 5P20GM103642-05), intramural funds of the National Institute of Drug Abuse, NSF (DBI-1337284), RCMI (NIMHD 8G12-MD007600), RISE Program (5R25GM061151), and grants from the Spanish “Ministerio de Economía y Competitividad” and European Regional Development Funds of the European Union (SAF2014-54840-R and SAF2017-87629-R), the “Fundació La Marató de TV3” (20140610), and Government of Catalonia (2017-SGR-1497).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sergi Ferré, Vicent Casadó, and Manuel Díaz-Ríos are co-senior authors.
Rights and permissions
About this article
Cite this article
Rivera-Oliver, M., Moreno, E., Álvarez-Bagnarol, Y. et al. Adenosine A1-Dopamine D1 Receptor Heteromers Control the Excitability of the Spinal Motoneuron. Mol Neurobiol 56, 797–811 (2019). https://doi.org/10.1007/s12035-018-1120-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1120-y