Abstract
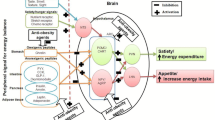
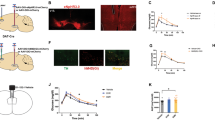
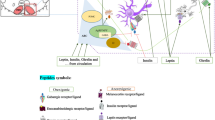
It is becoming increasingly apparent the importance of the central nervous system (CNS) as the major contributor to the regulation of systemic metabolism. Antipsychotic drugs are used often to treat several psychiatric disorders, including schizophrenia and bipolar disorder However, antipsychotic drugs prescription, particularly the second-generation ones (SGAs), such as clozapine and olanzapine, is related to a considerable weight gain which usually leads to obesity. The aim of this paper is to assess the influence of orexin A on sympathetic and hyperthermic reactions to several neuroleptic drugs. Orexin A is a neuropeptide which effects both body temperature and food intake by increasing sympathetic activity. Orexin A-mediated hyperthermia is reduced by haloperidol and is blocked by clozapine and olanzapine. Orexin A-mediated body temperature elevation is increased by risperidone. These hyperthermic effects are delayed by quietapine. In this paper, it is discussed the orexinergic pathway activation by neuroleptic drugs and its influence on human therapeutic strategies. With the aim to determine that neuroleptic drugs mediate body temperature control through to the orexinergic system, we summarized our previously published data. Psychiatric disorders increase the risk of developing metabolic disorders (e.g., weight gain, increased blood pressure, and glucose or lipid levels). Therefore, the choice of antipsychotic drug to be prescribed, based on the relevant risks and benefits of each individual drug, has an essential role in human health prevention.


Similar content being viewed by others
References
Bruce KD, Zsombok A, Eckel RH (2017) Lipid processing in the brain: a key regulator of systemic metabolism. Frontiers in Endocrinology, 8 (APR), art. no. 60. https://doi.org/10.3389/fendo.2017.00060
Messina G, Valenzano A, Moscatelli F, Salerno M, Lonigro A, Esposito T, Monda V, Corso G et al (2017) Role of autonomic nervous system and orexinergic system on adipose tissue. Frontiers in Physiology 8:137. https://doi.org/10.3389/fphys.2017.00137
Monda M, Viggiano A, Viggiano A, Mondola R, Viggiano E, Messina G, Tafuri D, De Luca V (2008) Olanzapine blocks the sympathetic and hyperthermic reactions due to cerebral injection of orexin. A Peptides 29(1):120–126. https://doi.org/10.1016/j.peptides.2007.10.016
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE et al (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327. https://doi.org/10.1073/pnas.95.1.322
Messina G, Vicidomini C, Viggiano A, Tafuri D, Cozza V, Cibelli G et al (2012) Enhanced parasympathetic activity of sportive women is paradoxically associated to enhanced resting energy expenditure. Auton Neurosci Basic Clin 169:102–106. https://doi.org/10.1016/j.autneu.2012.05.003
Wolf G (1998) Orexins: a newly discovered family of hypothalamic regulators of food intake. Nutr Rev 56:172–189
Grimaldi D, Silvani A, Benarroch EE, Cortelli P (2014) Orexin/hypocretin system and autonomic control: new insights and clinical correlations. Neurology 82:271–278. https://doi.org/10.1212/WNL.0000000000000045
Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D et al (2007) Sympathetic and hyperthermic reactions by orexin A: role of cerebral catecholaminergic neurons. Regul Pept 139:39–44. https://doi.org/10.1016/j.regpep.2006.10.002
Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC (1999) Cardiovascular regulatory actions of the hypocretins in brain. Brain Res 831:248–253. https://doi.org/10.1016/S0006-8993(99)01457-2
Monda M, Messina G, Mangoni C, De Luca B (2008) Resting energy expenditure and fat-free mass do not decline during aging in severely obese women. Clin Nutr 27:657–659. https://doi.org/10.1016/j.clnu.2008.04.005
Messina G, Viggiano A, De Luca V, Messina A, Chieffi S, Monda M (2013) Hormonal changes in menopause and orexin-A action. Obstet Gynecol Int 2013:209812. https://doi.org/10.1155/2013/209812
Messina G, Dalia C, Tafuri D, Monda V, Palmieri F, Dato A, et al. (2014). Orexin-A controls sympathetic activity and eating behavior. Front Psychol 5. doi:https://doi.org/10.3389/fpsyg.2014.00997.
Pilowsky PM, Lung MSY, Spirovski D, McMullan S (2009) Differential regulation of the central neural cardiorespiratory system by metabotropic neurotransmitters. Philos Trans R Soc B Biol Sci 364:2537–2552. https://doi.org/10.1098/rstb.2009.0092
Shahid IZ, Rahman AA, Pilowsky PM (2012) Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol 165:2292–2303. https://doi.org/10.1111/j.1476-5381.2011.01694.x
Tupone D, Madden CJ, Cano G, Morrison SF (2011) An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci 31:15944–15955. https://doi.org/10.1523/JNEUROSCI.3909-11.2011
Messina G, Monda V, Moscatelli F, Valenzano AA, Monda G, Esposito T et al (2015) Role of orexin system in obesity. Biol Med 7:1–6. https://doi.org/10.4172/0974-8369.1000248
Messina A, De Fusco C, Monda V, Esposito M, Moscatelli F, Valenzano A et al (2016) Role of the orexin system on the hypothalamus-pituitary-thyroid axis. Front Neural Circuits 10:66. https://doi.org/10.3389/fncir.2016.00066
Messina G, Di Bernardo G, Viggiano A, De Luca V, Monda V, Messina A et al (2016) Exercise increases the level of plasma orexin A in humans. J Basic Clin Physiol Pharmacol 27:611–616. https://doi.org/10.1515/jbcpp-2015-0133
Francavilla G, Abrignani MG, Braschi A, Sciacca R, Francavilla VC, Caracciolo MM et al (2007) Physical exercise and sport activities in patients with and without coronary heart disease. Monaldi ArchChest Dis 68:87–95
Francavilla VC, Abricnani M, Braschi A, Francavilla C (2008) Utility of QT dispersion in sports medicine. Medicina dello Sport 61:477–485
Kukkonen JP (2012). Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. AJP Cell Physiol, 2–32. doi:https://doi.org/10.1152/ajpcell.00227.2012.
Beuckmann CT, Yanagisawa M (2002) Orexins: from neuropeptides to energy homeostasis and sleep/wake regulation. J Mol Med (Berl) 80:329–342. https://doi.org/10.1007/s00109-002-0322-x
de Koning P, de Vries M (1995) A comparison of the neuro-endocrinological and temperature effects of DU 29894, flesinoxan, sulpiride and haloperidol in normal volunteers. Br J Clin Pharmacol 39:7–14. https://doi.org/10.1111/j.1365-2125.1995.tb04403.x
Halloran LL, Bernard DW (2004) Management of drug-induced hyperthermia. Curr Opin Pediatr 16:211–215. https://doi.org/10.1097/00008480-200404000-00018
He J, Xu H, Yang Y, Zhang X, Li XM (2004) Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of Bcl-2 decrease in rats. Brain Res 1018:186–192. https://doi.org/10.1016/j.brainres.2004.05.060
Razaq M, Samma M (2004) A case of risperidone-induced hypothermia. Am J Ther 11:229–230. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med5&NEWS=N&AN=15133539
Wenthur CJ (2016) Classics in chemical neuroscience: methylphenidate. ACS Chem Neurosci 7:1030–1040. https://doi.org/10.1021/acschemneuro.6b00199
Monda M, Viggiano A, De Luca V (2003) Haloperidol reduces the sympathetic and thermogenic activation induced by orexin A. Neurosci Res 45:17–23. https://doi.org/10.1016/S0168-0102(02)00191-8
Monda M, Viggiano AN, Viggiano AL, Viggiano E, De Luca V (2006) Risperidone potentiates the sympathetic and hyperthermic reactions induced by orexin A in the rat. Physiol Res 55:73–78
Monda M, Viggiano A, Viggiano A, Fuccio F, De Luca V (2004) Clozapine blocks sympathetic and thermogenic reactions induced by orexin A in rat. Physiol Res 53:507–513 Available at: http://www.ncbi.nlm.nih.gov/pubmed/15479129
Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D et al (2006) Quetiapine lowers sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Neuropeptides 40:357–363. https://doi.org/10.1016/j.npep.2006.07.003
Irving CB, Adams CE, Lawrie S (2009) Haloperidol versus placebo for schizophrenia. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003082.pub2
Kulkarni SK, Ninan I (2000) Dopamine D4 receptors and development of newer antipsychotic drugs. Fundam Clin Pharmacol 14:529–539 Available at: pm:11206702
Gobbi G, Janiri L (1999) Clozapine blocks dopamine, 5-HT2 and 5-HT3 responses in the medial prefrontal cortex: an in vivo microiontophoretic study. Eur Neuropsychopharmacol 10:43–49. https://doi.org/10.1016/S0924-977X(99)00055-3
Brunello N, Masotto C, Steardo L, Markstein R, Racagni G (1995) New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharmacology 13:223–234. https://doi.org/10.1016/0893-133X(95)00068-O
Bramanti V, Tomassoni D, Grasso S, Bronzi D, Napoli M, Campisi A, Li Volti G, Ientile R et al (2012) Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res 37(12):2795–2804. https://doi.org/10.1007/s11064-012-0873-3
Avola R, Di Tullio MA, Fisichella A, Tayebati SK, Tomassoni D (2004) Glial fibrillary acidic protein and vimentin expression is regulated by glucocorticoids and neurotrophic factors in primary rat astroglial cultures. Clin Exp Hypertens 26(4):323–333. https://doi.org/10.1081/CEH-120034137
McIntyre RS, McCann SM, Kennedy SH (2001) Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatr 46:273–281. https://doi.org/10.1177/070674370104600308
Bray GA (2000) Reciprocal relation of food intake and sympathetic activity: experimental observations and clinical implications. Int J Obes 24:S8–S17 Available at: d:%5CPDF%5C0244.pdf
Messina G, De Luca V, Viggiano A, Ascione A, Iannaccone T, Chieffi S, et al. (2013). Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol Res Int, 1–5. doi:https://doi.org/10.1155/2013/639280.
Monda M, Sullo A, De Luca B (1997) Lesions of the ventromedial hypothalamus reduce postingestional thermogenesis. Physiol Behav 61:687–691. https://doi.org/10.1016/S0031-9384(96)00520-3
Tauscher J, Hussain T, Agid O, Verhoeff NPLG, Wilson AA, Houle S et al (2004) Equivalent occupancy of dopamine D1 and D2 receptors, with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry 161:1620–1625. https://doi.org/10.1176/appi.ajp.161.9.1620
Bishara D, Olofinjana O, Sparshatt A, Kapur S, Taylor D, Patel MX (2013) Olanzapine: a systematic review and meta-regression of the relationships between dose, plasma concentration, receptor occupancy, and response. J Clin Psychopharmacol 33:329–335. https://doi.org/10.1097/JCP.0b013e31828b28d5
Uchida S, Kato Y, Hirano K, Kagawa Y, Yamada S (2007) Brain neurotransmitter receptor-binding characteristics in rats after oral administration of haloperidol, risperidone and olanzapine. Life Sci 80:1635–1640. https://doi.org/10.1016/j.lfs.2007.01.038
Lee TW, Tsai SJ, Hwang JP (2003) Severe cardiovascular side effects of olanzapine in an elderly patient: case report. Int J Psychiatry Med 33:399–401 Available at: 15152790
Milano W, Grillo F, Del Mastro A, De Rosa M, Sanseverino B, Petrella C et al (2007) Appropriate intervention strategies for weight gain induced by olanzapine: a randomized controlled study. Adv Ther 24:123–134. https://doi.org/10.1007/BF02850000
De Hert M, Schreurs V, Sweers K, Van Eyck D, Hanssens L, Šinko S et al (2008) Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res 101:295–303. https://doi.org/10.1016/j.schres.2008.01.028
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO et al (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. https://doi.org/10.1056/NEJMoa1203165
Eder U, Mangweth B, Ebenbichler C, Weiss E, Hofer A, Hummer M et al (2001) Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry 158:1719–1722. https://doi.org/10.1176/appi.ajp.158.10.1719
Wirshing DA, Spellberg BJ, Erhart SM, Marder SR, Wirshing WC (1998) Novel antipsychotics and new onset diabetes. Biol Psychiatry 44:778–783. https://doi.org/10.1016/S0006-3223(98)00100-0
Ota M, Mori K, Nakashima A, Kaneko YS, Fujiwara K, Itoh M et al (2002) Peripheral injection of risperidone, an atypical antipsychotic, alters the bodyweight gain of rats. Clin Exp Pharmacol Physiol 29:980–989. https://doi.org/10.1046/j.1440-1681.2002.t01-1-03755.x
Morrison SF (2004) Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci 19:67–74. https://doi.org/10.1152/nips.01502.2003
Oerther S, Ahlenius S (2000) Atypical antipsychotics and dopamine D(1) receptor agonism: an in vivo experimental study using core temperature measurements in the rat. J Pharmacol Exp Ther 292:731–736. http://jpet.aspetjournals.org/content/292/2/731.abstract
Suttajit S, Srisurapanont M, Maneeton B, Maneeton N, Suttajit S (2009) Quetiapine versus typical antipsychotic medications for schizophrenia. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007815
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359. https://doi.org/10.1152/physrev.00015.2003
Li X, Johnson MS, Smith DL, Li Y, Kesterson RA, Allison DB et al (2013) Effects of risperidone on energy balance in female C57BL/6J mice. Obesity 21:1850–1857. https://doi.org/10.1002/oby.20350
Girault EM, Foppen E, Ackermans MT, Fliers E, Kalsbeek A (2013) Central administration of an orexin receptor 1 antagonist prevents the stimulatory effect of Olanzapine on endogenous glucose production. Brain Res 1527:238–245. https://doi.org/10.1016/j.brainres.2013.06.034
Monda M, Viggiano A, De Luca V (2003) Paradoxical [correction of parodoxical] effect of orexin A: hypophagia induced by hyperthermia. Brain Res 961:220–228 http://www.ncbi.nlm.nih.gov/pubmed/12531489
Tiwari AK, Brandl EJ, Zai CC, Goncalves VF, Chowdhury NI, Freeman N et al (2015) Association of orexin receptor polymorphisms with antipsychotic-induced weight gain. World J Biol Psychiatry 2975:1–9. https://doi.org/10.3109/15622975.2015.1076173
Krystal AD (2015) New developments in insomnia medications of relevance to mental health disorders. Psychiatr Clin North Am 38:843–860. https://doi.org/10.1016/j.psc.2015.08.001
Melnyk A, Harper ME, Himms-Hagen J (1997) Raising at thermoneutrality prevents obesity and hyperphagia in BAT-ablated transgenic mice. Am. J. Physiol 272:R1088–R1093 Available at: http://www.ncbi.nlm.nih.gov/pubmed/9140006
Majercikova Z, Kiss A (2016) Stress alters asenapine-induced Fos expression in the Meynert’s nucleus: response of adjacent hypocretin and melanin-concentrating hormone neurons in rat. Neurol Res 38:32–39. https://doi.org/10.1080/01616412.2015.1105585
Protopopova D, Masopust J, Maly R, Valis M, Bazant J (2012) The prevalence of cardiometabolic risk factors and the ten-year risk of fatal cardiovascular events in patients with schizophrenia and related psychotic disorders. Psychiatr Danub 24:307–313
Kraal AZ, Ward KM, Ellingrod VL (2017) Sex differences in antipsychotic related metabolic functioning in schizophrenia spectrum disorders. Psychopharmacol Bull 47(2):8–21
Miyauchi M, Kishida I, Suda A, Shiraishi Y, Fujibayashi M, Taguri M, Ishii C, Ishii N et al (2017) Long term effects of smoking cessation in hospitalized schizophrenia patients. BMC Psychiatry 17(1):87. https://doi.org/10.1186/s12888-017-1250-1
Salomone F, Li Volti G, Vitaglione P, Morisco F, Fogliano V, Zappalà A, Palmigiano A, Garozzo D et al (2014) Coffee enhances the expression of chaperones and antioxidant proteins in rats with nonalcoholic fatty liver disease. Transl Res 163(6):593–602. https://doi.org/10.1016/j.trsl.2013.12.001
Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, Koyuncu K, Schjerning O et al (2017) Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry 74(7):719–728. https://doi.org/10.1001/jamapsychiatry.2017.1220
Reeves R, Tamburello A, DeBilio L (2017) Metabolic syndrome prevalence and reduction in inmates prescribed antipsychotic medications. Journal of Correctional Health Care 23(2):203–213. https://doi.org/10.1177/1078345817700802
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monda, V., Salerno, M., Sessa, F. et al. Functional Changes of Orexinergic Reaction to Psychoactive Substances. Mol Neurobiol 55, 6362–6368 (2018). https://doi.org/10.1007/s12035-017-0865-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0865-z