Abstract
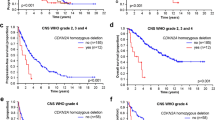
Glioblastoma (GBM) is an aggressive primary brain tumor. The rapid growth and the privileged provenance of the tumor within the brain contribute to its aggressivity and poor therapeutic targeting. A poor prognostic factor in glioblastoma is the deletion or mutation of the Nf1 gene. This gene codes for the protein neurofibromin, a tumor suppressor gene that is known to interact with the collapsin response mediator protein 2 (CRMP2). CRMP2 expression and elevated expression of nuclear phosphorylated CRMP2 have recently been implicated in cancer progression. The CRMP2-neurofibromin interaction protects CRMP2 from its phosphorylation by cyclin-dependent kinase 5 (Cdk5), an event linked to cancer progression. In three human glioblastoma cell lines (GL15, A172, and U87), we observed an inverse correlation between neurofibromin expression and CRMP2 phosphorylation levels. Glioblastoma cell proliferation was dependent on CRMP2 expression and phosphorylation by Cdk5 and glycogen synthase kinase 3 beta (GSK3β). The CRMP2 phosphorylation inhibitor (S)-lacosamide reduces, in a concentration-dependent manner, glioblastoma cell proliferation and induced apoptosis in all three GBM cell lines tested. Since (S)-lacosamide is bioavailable in the brain, we tested its utility in an in vivo orthotopic model of GBM using GL261-LucNeo glioma cells. (S)-lacosamide decreased tumor size, as measured via in vivo bioluminescence imaging, by ~54% compared to vehicle control. Our results introduce CRMP2 expression and phosphorylation as a novel player in GBM proliferation and survival, which is enhanced by loss of Nf1.







Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. doi:10.1056/NEJMoa043330
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D et al (2013) The somatic genomic landscape of glioblastoma. Cell 155(2):462–477. doi:10.1016/j.cell.2013.09.034
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17(1):98–110. doi:10.1016/j.ccr.2009.12.020
Bianco J, Bastiancich C, Jankovski A, des Rieux A, Preat V, Danhier F (2017) On glioblastoma and the search for a cure: where do we stand? Cell Mol Life Sci. doi:10.1007/s00018-017-2483-3
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812. doi:10.1126/science.1164382
Sintupisut N, Liu PL, Yeang CH (2013) An integrative characterization of recurrent molecular aberrations in glioblastoma genomes. Nucleic Acids Res 41(19):8803–8821. doi:10.1093/nar/gkt656
Plaisier CL, O’Brien S, Bernard B, Reynolds S, Simon Z, Toledo CM, Ding Y, Reiss DJ et al (2016) Causal mechanistic regulatory network for glioblastoma deciphered using systems genetics Network analysis. Cell Syst 3(2):172–186. doi:10.1016/j.cels.2016.06.006
Wood MD, Reis GF, Reuss DE, Phillips JJ (2016) Protein analysis of glioblastoma primary and posttreatment pairs suggests a mesenchymal shift at recurrence. J Neuropathol Exp Neurol 75(10):925–935. doi:10.1093/jnen/nlw068
Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD et al (2012) VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22(1):21–35. doi:10.1016/j.ccr.2012.05.037
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C et al (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A 110(10):4009–4014. doi:10.1073/pnas.1219747110
Ballester R, Marchuk D, Boguski M, Saulino A, Letcher R, Wigler M, Collins F (1990) The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell 63(4):851–859
McGillicuddy LT, Fromm JA, Hollstein PE, Kubek S, Beroukhim R, De Raedt T, Johnson BW, Williams SM et al (2009) Proteasomal and genetic inactivation of the NF1 tumor suppressor in gliomagenesis. Cancer Cell 16(1):44–54. doi:10.1016/j.ccr.2009.05.009
Zuckermann M, Hovestadt V, Knobbe-Thomsen CB, Zapatka M, Northcott PA, Schramm K, Belic J, Jones DT et al (2015) Somatic CRISPR/Cas9-mediated tumour suppressor disruption enables versatile brain tumour modelling. Nat Commun 6:7391. doi:10.1038/ncomms8391
Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM (1995) Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature 376(6540):509–514. doi:10.1038/376509a0
Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N et al (2002) CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol 4(8):583–591. doi:10.1038/ncb825
Ip JP, Fu AK, Ip NY (2014) CRMP2: functional roles in neural development and therapeutic potential in neurological diseases. Neuroscientist. doi:10.1177/1073858413514278
Patrakitkomjorn S, Kobayashi D, Morikawa T, Wilson MM, Tsubota N, Irie A, Ozawa T, Aoki M et al (2008) Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2. J Biol Chem 283(14):9399–9413. doi:10.1074/jbc.M708206200
Grant NJ, Coates PJ, Woods YL, Bray SE, Morrice NA, Hastie CJ, Lamont DJ, Carey FA et al (2015) Phosphorylation of a splice variant of collapsin response mediator protein 2 in the nucleus of tumour cells links cyclin dependent kinase-5 to oncogenesis. BMC Cancer 15:885. doi:10.1186/s12885-015-1691-1
Tan F, Thiele CJ, Li Z (2014) Collapsin response mediator proteins: potential diagnostic and prognostic biomarkers in cancers (review). Oncol Lett 7(5):1333–1340. doi:10.3892/ol.2014.1909
Shimada K, Ishikawa T, Nakamura F, Shimizu D, Chishima T, Ichikawa Y, Sasaki T, Endo I et al (2014) Collapsin response mediator protein 2 is involved in regulating breast cancer progression. Breast Cancer 21(6):715–723. doi:10.1007/s12282-013-0447-5
Oliemuller E, Pelaez R, Garasa S, Pajares MJ, Agorreta J, Pio R, Montuenga LM, Teijeira A et al (2013) Phosphorylated tubulin adaptor protein CRMP-2 as prognostic marker and candidate therapeutic target for NSCLC. Int J Cancer 132(9):1986–1995. doi:10.1002/ijc.27881
Mukherjee J, DeSouza LV, Micallef J, Karim Z, Croul S, Siu KW, Guha A (2009) Loss of collapsin response mediator Protein1, as detected by iTRAQ analysis, promotes invasion of human gliomas expressing mutant EGFRvIII. Cancer Res 69(22):8545–8554. doi:10.1158/0008-5472.CAN-09-1778
Moutal A, Honnorat J, Massoma P, Desormeaux P, Bertrand C, Malleval C, Watrin C, Chounlamountri N et al (2015) CRMP5 controls glioblastoma cell proliferation and survival through notch-dependent signaling. Cancer Res 75(17):3519–3528. doi:10.1158/0008-5472.CAN-14-0631
Wilson SM, Xiong W, Wang Y, Ping X, Head JD, Brittain JM, Gagare PD, Ramachandran PV et al (2012) Prevention of posttraumatic axon sprouting by blocking collapsin response mediator protein 2-mediated neurite outgrowth and tubulin polymerization. Neuroscience 210:451–466. doi:10.1016/j.neuroscience.2012.02.038
Moutal A, Eyde N, Telemi E, Park KD, Xie JY, Dodick DW, Porreca F, Khanna R (2016) Efficacy of (S)-lacosamide in preclinical models of cephalic pain. Pain Rep 1(1). doi:10.1097/PR9.0000000000000565
Moutal A, Chew LA, Yang X, Wang Y, Yeon SK, Telemi E, Meroueh S, Park KD et al (2016) (S)-lacosamide inhibition of CRMP2 phosphorylation reduces postoperative and neuropathic pain behaviors through distinct classes of sensory neurons identified by constellation pharmacology. Pain 157(7):1448–1463. doi:10.1097/j.pain.0000000000000555
Moutal A, Francois-Moutal L, Perez-Miller S, Cottier K, Chew LA, Yeon SK, Dai J, Park KD et al (2016) (S)-lacosamide binding to collapsin response mediator protein 2 (CRMP2) regulates CaV2.2 activity by subverting its phosphorylation by Cdk5. Mol Neurobiol 53(3):1959–1976. doi:10.1007/s12035-015-9141-2
Bocchini V, Casalone R, Collini P, Rebel G, Lo Curto F (1991) Changes in glial fibrillary acidic protein and karyotype during culturing of two cell lines established from human glioblastoma multiforme. Cell Tissue Res 265(1):73–81
Clark AJ, Safaee M, Oh T, Ivan ME, Parimi V, Hashizume R, Ozawa T, James CD et al (2014) Stable luciferase expression does not alter immunologic or in vivo growth properties of GL261 murine glioma cells. J Transl Med 12:345. doi:10.1186/s12967-014-0345-4
Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R (2009) An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem 284(45):31375–31390. doi:10.1074/jbc.M109.009951
Dustrude ET, Moutal A, Yang X, Wang Y, Khanna M, Khanna R (2016) Hierarchical CRMP2 posttranslational modifications control NaV1.7 function. Proc Natl Acad Sci U S A 113(52):E8443–E8452. doi:10.1073/pnas.1610531113
Choi D, Stables JP, Kohn H (1996) Synthesis and anticonvulsant activities of N-benzyl-2-acetamidopropionamide derivatives. J Med Chem 39(9):1907–1916
Francois-Moutal L, Wang Y, Moutal A, Cottier KE, Melemedjian OK, Yang X, Wang Y, Ju W et al (2015) A membrane-delimited N-myristoylated CRMP2 peptide aptamer inhibits CaV2.2 trafficking and reverses inflammatory and postoperative pain behaviors. Pain 156(7):1247–1264. doi:10.1097/j.pain.0000000000000147
Dustrude ET, Wilson SM, Ju W, Xiao Y, Khanna R (2013) CRMP2 protein SUMOylation modulates NaV1.7 channel trafficking. J Biol Chem 288(34):24316–24331. doi:10.1074/jbc.M113.474924
Cook RL, Householder KT, Chung EP, Prakapenka AV, DiPerna DM, Sirianni RW (2015) A critical evaluation of drug delivery from ligand modified nanoparticles: Confounding small molecule distribution and efficacy in the central nervous system. J Control Release 220(Pt A):89–97. doi:10.1016/j.jconrel.2015.10.013
Abdelwahab MG, Sankar T, Preul MC, Scheck AC (2011) Intracranial implantation with subsequent 3D in vivo bioluminescent imaging of murine gliomas. J Vis Exp 57:e3403. doi:10.3791/3403
Householder KT, DiPerna DM, Chung EP, Wohlleb GM, Dhruv HD, Berens ME, Sirianni RW (2015) Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int J Pharm 479(2):374–380. doi:10.1016/j.ijpharm.2015.01.002
Cole AR, Knebel A, Morrice NA, Robertson LA, Irving AJ, Connolly CN, Sutherland C (2004) GSK-3 phosphorylation of the Alzheimer epitope within collapsin response mediator proteins regulates axon elongation in primary neurons. J Biol Chem 279(48):50176–50180. doi:10.1074/jbc.C400412200
Koo TS, Kim SJ, Ha DJ, Baek M, Moon H (2011) Pharmacokinetics, brain distribution, and plasma protein binding of the antiepileptic drug lacosamide in rats. Arch Pharm Res 34(12):2059–2064. doi:10.1007/s12272-011-1208-7
Elizabeth W. Newcomb DZ (2009) The murine GL261 glioma experimental model to assess novel brain tumor treatments. In: Erwin G. Van Meir P (ed) Models, Markers, Prognostic Factors, Targets, and Therapeutic Approaches. Humana Press. 227-241. doi:10.1007/978-1-60327-553-8_12
Toledo CM, Ding Y, Hoellerbauer P, Davis RJ, Basom R, Girard EJ, Lee E, Corrin P et al (2015) Genome-wide CRISPR-Cas9 screens reveal loss of redundancy between PKMYT1 and WEE1 in glioblastoma stem-like cells. Cell Rep 13(11):2425–2439. doi:10.1016/j.celrep.2015.11.021
Cole AR, Causeret F, Yadirgi G, Hastie CJ, McLauchlan H, McManus EJ, Hernandez F, Eickholt BJ et al (2006) Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. J Biol Chem 281(24):16591–16598. doi:10.1074/jbc.M513344200
Cole AR, Soutar MP, Rembutsu M, van Aalten L, Hastie CJ, McLauchlan H, Peggie M, Balastik M et al (2008) Relative resistance of Cdk5-phosphorylated CRMP2 to dephosphorylation. J Biol Chem 283(26):18227–18237. doi:10.1074/jbc.M801645200
Balastik M, Zhou XZ, Alberich-Jorda M, Weissova R, Ziak J, Pazyra-Murphy MF, Cosker KE, Machonova O et al (2015) Prolyl isomerase Pin1 regulates axon guidance by stabilizing CRMP2A selectively in distal axons. Cell Rep 13(4):812–828. doi:10.1016/j.celrep.2015.09.026
Kamiya Y, Saeki K, Takiguchi M, Funakoshi K (2013) CDK5, CRMP2 and NR2B in spinal dorsal horn and dorsal root ganglion have different role in pain signaling between neuropathic pain model and inflammatory pain model. Eur J Anaesthesiol 30:214–214
Korur S, Huber RM, Sivasankaran B, Petrich M, Morin P Jr, Hemmings BA, Merlo A, Lino MM (2009) GSK3beta regulates differentiation and growth arrest in glioblastoma. PLoS One 4(10):e7443. doi:10.1371/journal.pone.0007443
Domoto T, Pyko IV, Furuta T, Miyashita K, Uehara M, Shimasaki T, Nakada M, Minamoto T (2016) Glycogen synthase kinase-3beta is a pivotal mediator of cancer invasion and resistance to therapy. Cancer Sci 107(10):1363–1372. doi:10.1111/cas.13028
Pyko IV, Nakada M, Sabit H, Teng L, Furuyama N, Hayashi Y, Kawakami K, Minamoto T et al (2013) Glycogen synthase kinase 3beta inhibition sensitizes human glioblastoma cells to temozolomide by affecting O6-methylguanine DNA methyltransferase promoter methylation via c-Myc signaling. Carcinogenesis 34(10):2206–2217. doi:10.1093/carcin/bgt182
Schiel JA, Park K, Morphew MK, Reid E, Hoenger A, Prekeris R (2011) Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J Cell Sci 124(Pt 9):1411–1424. doi:10.1242/jcs.081448
Fremont S, Romet-Lemonne G, Houdusse A, Echard A (2017) Emerging roles of MICAL family proteins—from actin oxidation to membrane trafficking during cytokinesis. J Cell Sci. doi:10.1242/jcs.202028
Brittain JM, Wang Y, Eruvwetere O, Khanna R (2012) Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS Lett 586(21):3813–3818. doi:10.1016/j.febslet.2012.09.022
Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K et al (2005) Semaphorin3A signalling is mediated via sequential Cdk5 and GSK3beta phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes Cells 10(2):165–179. doi:10.1111/j.1365-2443.2005.00827.x
Schmidt EF, Shim SO, Strittmatter SM (2008) Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein. J Neurosci 28(9):2287–2297. doi:10.1523/JNEUROSCI.5646-07.2008
Bagci T, Wu JK, Pfannl R, Ilag LL, Jay DG (2009) Autocrine semaphorin 3A signaling promotes glioblastoma dispersal. Oncogene 28(40):3537–3550. doi:10.1038/onc.2009.204
Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP (2000) Numb is an endocytic protein. J Cell Biol 151(6):1345–1352
See WL, Tan IL, Mukherjee J, Nicolaides T, Pieper RO (2012) Sensitivity of glioblastomas to clinically available MEK inhibitors is defined by neurofibromin 1 deficiency. Cancer Res 72(13):3350–3359. doi:10.1158/0008-5472.CAN-12-0334
Han S, Meng L, Jiang Y, Cheng W, Tie X, Xia J, Wu A (2017) Lithium enhances the antitumour effect of temozolomide against TP53 wild-type glioblastoma cells via NFAT1/FasL signalling. Br J Cancer. doi:10.1038/bjc.2017.89
Adamo A, Fiore D, De Martino F, Roscigno G, Affinito A, Donnarumma E, Puoti I, Ricci-Vitiani L et al (2017) RYK promotes the stemness of glioblastoma cells via the WNT/ beta-catenin pathway. Oncotarget. doi:10.18632/oncotarget.14564
Wu A, Oh S, Wiesner SM, Ericson K, Chen L, Hall WA, Champoux PE, Low WC et al (2008) Persistence of CD133+ cells in human and mouse glioma cell lines: detailed characterization of GL261 glioma cells with cancer stem cell-like properties. Stem Cells Dev 17(1):173–184. doi:10.1089/scd.2007.0133
Moutal A, Chew LA, Yang X, Wang Y, Yeon SK, Telemi E, Meroueh S, Park KD et al (2016) (S)-lacosamide inhibition of CRMP2 phosphorylation reduces postoperative and neuropathic pain behaviors through distinct classes of sensory neurons identified by constellation pharmacology. Pain. doi:10.1097/j.pain.0000000000000555
Acknowledgements
This work was supported by a Neurofibromatosis New Investigator Award from the Department of Defense Congressionally Directed Military Medical Research and Development Program (NF1000099) and a Children’s Tumor Foundation NF1 Synodos award to R.K. A.M. was supported by a Young Investigator’s Award from the Children’s Tumor Foundation. L.S.V. was supported by grants from a T35 HL07479-31A1 training grant from the NIH/NHLBI to Marlys H. Witte (Department of Surgery, University of Arizona) as well as a grant from the Undergraduate Biology Research Program (UBRP), University of Arizona and Western Alliance to Expand Student Opportunities (WAESO) Louis Stokes Alliance for Minority Participation (LSAMP) National Science Foundation (NSF) Cooperative Agreement No. HRD-1101728. Further support was provided by Arizona State University (K.T.H.) and the Barrow Neurological Foundation (K.T.H., R.W.S.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Moutal, A., Villa, L.S., Yeon, S.K. et al. CRMP2 Phosphorylation Drives Glioblastoma Cell Proliferation. Mol Neurobiol 55, 4403–4416 (2018). https://doi.org/10.1007/s12035-017-0653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0653-9