Abstract
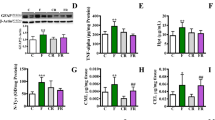
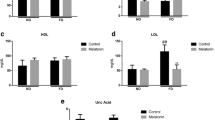
The drastic increase in the consumption of fructose encouraged the research to focus on its effects on brain physio-pathology. Although young and adults differ largely by their metabolic and physiological profiles, most of the previous studies investigated brain disturbances induced by long-term fructose feeding in adults. Therefore, we investigated whether a short-term consumption of fructose (2 weeks) produces early increase in specific markers of inflammation and oxidative stress in the hippocampus of young and adult rats. After the high-fructose diet, plasma lipopolysaccharide and tumour necrosis factor (TNF)-alpha were found significantly increased in parallel with hippocampus inflammation, evidenced by a significant rise in TNF-alpha and glial fibrillar acidic protein concentrations in both the young and adult groups. The fructose-induced inflammatory condition was associated with brain oxidative stress, as increased levels of lipid peroxidation and nitro-tyrosine were detected in the hippocampus. The degree of activation of the protein kinase B, extracellular signal-regulated kinase 1/2, and insulin receptor substrate 1 pathways found in the hippocampus after fructose feeding indicates that the detrimental effects of the fructose-rich diet might largely depend on age. Mitochondrial function in the hippocampus, together with peroxisome proliferator-activated receptor gamma coactivator 1-alpha content, was found significantly decreased in fructose-treated adult rats. In vitro studies with BV-2 microglial cells confirmed that fructose treatment induces TNF-alpha production as well as oxidative stress. In conclusion, these results suggest that unbalanced diet, rich in fructose, may be highly deleterious in young people as in adults and must be strongly discouraged for the prevention of diet-associated neuroinflammation and neurological diseases.








Similar content being viewed by others
References
Campos VC, Tappy L (2016) Physiological handling of dietary fructose-containing sugars: implications for health. Int J Obes Suppl 1:S6–11. doi:10.1038/ijo.2016.8
Stanhope KL (2016) Sugar consumption, metabolic disease and obesity: the state of the controversy. Crit Rev Clin Lab Sci 53:52–67. doi:10.3109/10408363.2015.1084990
Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332:145–159
Crescenzo R, Bianco F, Falcone I, Coppola P, Liverini G, Iossa S (2013) Increased hepatic de novo lipogenesis and mitochondrial efficiency in a model of obesity induced by diets rich in fructose. Eur J Nutr 52:537–545. doi:10.1007/s00394-012-0356-y
Crescenzo R, Bianco F, Coppola P, Mazzoli A, Cigliano L, Liverini G, Iossa S (2013) Increased skeletal muscle mitochondrial efficiency in rats with fructose-induced alteration in glucose tolerance. Br J Nutr 110:1996–2003. doi:10.1017/S0007114513001566
Crescenzo R, Bianco F, Coppola P, Mazzoli A, Valiante S, Liverini G, Iossa S (2014) Adipose tissue remodeling in rats exhibiting fructose-induced obesity. Eur J Nutr 53:413–419. doi:10.1007/s00394-013-0538-2
Crescenzo R, Bianco F, Coppola P, Mazzoli A, Tussellino M, Carotenuto R, Liverini G, Iossa S (2014) Fructose supplementation worsens the deleterious effects of short-term high-fat feeding on hepatic steatosis and lipid metabolism in adult rats. Exp Physiol 99:1203–1213. doi:10.1113/expphysiol.2014.079632
Crescenzo R, Bianco F, Coppola P, Mazzoli A, Cigliano L, Liverini G, Iossa S (2015) The effect of high-fat--high-fructose diet on skeletal muscle mitochondrial energetics in adult rats. Eur J Nutr 54:183–192. doi:10.1007/s00394-014-0699-7
Van der Borght K, Köhnke R, Göransson N, Deierborg T, Brundin P, Erlanson-Albertsson C, Lindqvist A (2011) Reduced neurogenesis in the rat hippocampus following high fructose consumption. Regul Pept 167:26–30. doi:10.1016/j.regpep.2010.11.002
Mastrocola R, Nigro D, Cento AS, Chiazza F, Collino M, Aragno M (2016) High-fructose intake as risk factor for neurodegeneration: key role for carboxymethyllysine accumulation in mice hippocampal neurons. Neurobiol Dis 89:65–75. doi:10.1016/j.nbd.2016.02.005
Li JM, Ge CX, Xu MX, Wang W, Yu R, Fan CY, Kong LD (2015) Betaine recovers hypothalamic neural injury by inhibiting astrogliosis and inflammation in fructose-fed rats. Mol Nutr Food Res 59:189–202. doi:10.1002/mnfr.201400307
Yin Q, Ma Y, Hong Y, Hou X, Chen J, Shen C, Sun M, Shang Y et al (2014) Lycopene attenuates insulin signaling deficits, oxidative stress, neuroinflammation, and cognitive impairment in fructose-drinking insulin resistant rats. Neuropharmacology 86:389–396. doi:10.1016/j.neuropharm.2014.07.020
Lopes A, Vilela TC, Taschetto L, Vuolo F, Petronilho F, Dal-Pizzol F, Streck EL, Ferreira GC et al (2014) Evaluation of the effects of fructose on oxidative stress and inflammatory parameters in rat brain. Mol Neurobiol 50:1124–1130. doi:10.1007/s12035-014-8676-y
Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC (2014) Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci 17:241–251. doi:10.1179/1476830513Y.0000000092
Ford CN, Slining MM, Popkin BM (2013) Trends in dietary intake among US 2- to 6-year-old children, 1989-2008. J Acad Nutr Diet 113:35–42. doi:10.1016/j.jand.2012.08.022
Cummings JL, Cole G (2002) Alzheimer disease. JAMA 287:2335–2338
Raskin J, Cummings J, Hardy J, Schuh K, Dean RA (2015) Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr Alzheimer Res 12(8):712–722
Spagnuolo MS, Mollica MP, Maresca B, Cavaliere G, Cefaliello C, Trinchese G, Scudiero R, Crispino M et al (2015) High fat diet and inflammation—modulation of haptoglobin level in rat brain. Front Cell Neurosci 9:479. doi:10.3389/fncel.2015.00479
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models Gastroenterol 87:1344–1350
Kim JJ, Shajib MS, Manocha MM, Khan WI (2012) Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 60:3678. doi:10.3791/3678
Fernandes MA, Custódio JB, Santos MS, Moreno AJ, Vicente JA (2006) Tetrandrine concentrations not affecting oxidative phosphorylation protect rat liver mitochondria from oxidative stress. Mitochondrion 6:176–185
Barré H, Bailly L, Rouanet JL (1987) Increased oxidative capacity in skeletal muscle from cold-acclimated ducklings: a comparison with rats. Comp Biochem Physiol 88B:5119–5522
Srere PA (1969) Citrate synthase. Meth Enzymol 13:3–11
Spagnuolo MS, Maresca B, Mollica MP, Cavaliere G, Cefaliello C, Trinchese G, Esposito MG, Scudiero R et al (2014) Haptoglobin increases with age in rat hippocampus and modulates apolipoprotein E mediated cholesterol trafficking in neuroblastoma cell lines. Front Cell Neurosci 8:212. doi:10.3389/fncel.2014.00212
Miller DB, O’Callaghan JP (2005) Aging, stress and the hippocampus. Ageing Res Rev 4:123–140
Lin S, Yang Z, Liu H, Tang L, Cai Z (2011) Beyond glucose: metabolic shifts in responses to the effects of the oral glucose tolerance test and the high-fructose diet in rats. Mol BioSyst 7:1537–1548. doi:10.1039/c0mb00246a
Madani Z, Malaisse WJ, Ait-Yahia D (2015) A comparison between the impact of two types of dietary protein on brain glucose concentrations and oxidative stress in high fructose-induced metabolic syndrome rats. Biomed Rep 3:731–735. doi:10.3390/nu3110987
Roberts RA, Smith RA, Safe S, Szabo C, Tjalkens RB, Robertson FM (2010) Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology 276:85–94. doi:10.1016/j.tox.2010.07.009
Holloszy JO, Oscai LB, Don IJ, Molé PA (1970) Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun 40(6):1368–1373
Hassel B, Elsais A, Froland AS, Tauboll E, Gjerstad L, Quan Y, Dingledine R, Rise F (2015) Uptake and metabolism of fructose by rat neocortical cells in vivo and by isolated nerve terminals in vitro. J Neurochem 133:572–581. doi:10.1111/jnc.13079
Xu MX, Yu R, Shao LF, Zhang YX, Ge CX, Liu XM, Wu WY, Li JM et al (2016) Up-regulated fractalkine (FKN) and its receptor CX3CR1 are involved in fructose-induced neuroinflammation: suppression by curcumin. Brain Behav Immun 58:69–81. doi:10.1016/j.bbi.2016.01.001
Stephan BC, Wells JC, Brayne C, Albanese E, Siervo M (2010) Increased fructose intake as a risk factor for dementia. J Gerontol A Biol Sci Med Sci 65:809–814. doi:10.1093/gerona/glq079
Hipkiss AR (2014) Aging risk factors and Parkinson’s disease: contrasting roles of common dietary constituents. Neurobiol Aging 35:1469–1472. doi:10.1016/j.neurobiolaging.2013.11.032
Hsu TM, Konanur VR, Taing L, Usui R, Kayser BD, Goran MI, Kanoski SE (2015) Effects of sucrose and high fructose corn syrup consumption on spatial memory function and hippocampal neuroinflammation in adolescent rats. Hippocampus 25:227–239. doi:10.1002/hipo.22368
Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M (2016) Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. doi:10.1093/gerona/glw240
Di Luccia B, Crescenzo R, Mazzoli A, Cigliano L, Venditti P, Walser JC, Widmer A, Baccigalupi L et al (2015) Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One 10:e0134893. doi:10.1371/journal.pone.0134893
Shu HJ, Isenberg K, Cormier RJ, Benz A, Zorumski CF (2006) Expression of fructose sensitive glucose transporter in the brains of fructose-fed rats. Neuroscience 140:889–895
Urrutia PJ, Mena NP, Núñez MT (2014) The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front Pharmacol 5:38 . doi:10.3389/fphar.2014.00038 eCollection 2014
Possel H, Noack H, Putzke J, Wolf G, Sies H (2000) Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia 32:51–59
Hewett JA, Hewett SJ (2012) Induction of nitric oxide synthase-2 expression and measurement of nitric oxide production in enriched primary cortical astrocyte cultures. Methods Mol Biol 814:251–263. doi:10.1007/978-1-61779-452-0_17
Chung KK, David KK (2010) Emerging roles of nitric oxide in neurodegeneration. Nitric Oxide 22:290–295. doi:10.1016/j.niox.2010.02.002
Malinski T (2007) Nitric oxide and nitroxidative stress in Alzheimer’s disease. J Alzheimers Dis 11:207–218
Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R (2007) Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res 1148:243–248
Butterfield DA, Reed T, Sultana R (2011) Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic Res 45:59–72. doi:10.3109/10715762.2010.520014
Subramaniam S, Unsicker K (2010) ERK and cell death: ERK1/2 in neuronal death. FEBS J 277:22–29. doi:10.1111/j.1742-4658.2009.07367.x
Zhang JZ, Jing L, Guo FY, Ma Y, Wang YL (2007) Inhibitory effect of ketamine on phosphorylation of the extracellular signal-regulated kinase 1/2 following brain ischemia and reperfusion in rats with hyperglycemia. Exp Toxicol Pathol 59:227–235
Frasca G, Carbonaro V, Merlo S, Copani A, Sortino MA (2008) Integrins mediate beta-amyloid-induced cell-cycle activation and neuronal death. J Neurosci Res 86:350–355
Bellaver B, Souza DG, Bobermin LD, Souza DO, Gonçalves CA, Quincozes-Santos A (2015) Resveratrol protects hippocampal astrocytes against LPS-induced neurotoxicity through HO-1, p38 and ERK pathways. Neurochem Res 40:1600–1608. doi:10.1007/s11064-015-1636-8
Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, Braak H, Tsujio I, Takeda M et al (2003) Role of protein kinase B in Alzheimer’s neurofibrillary pathology. Acta Neuropathol (Berl) 105:381–392
Rickle A, Bogdanovic N, Volkman I, Winblad B, Ravid R, Cowburn RF (2004) Akt activity in Alzheimer’s disease and other neurodegenerative disorders. Neuroreport 15:955–959
Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C (2005) Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem 93:105–117. doi:10.1111/j.1471-4159.2004.02949.x
Lee J, Boo JH, Ryu H (2009) The failure of mitochondria leads to neurodegeneration: do mitochondria need a jump start? Adv Drug Deliv Rev 61:1316–1323. doi:10.1016/j.addr.2009.07.016
Young-Collier KJ, McArdle M, Bennett JP (2012) The dying of the light: mitochondrial failure in Alzheimer’s disease. J Alzheimers Dis 28:771–781. doi:10.3233/JAD-2011-111487
Butterfield DA, Perluigi M, Sultana R (2006) Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol 545:39–50
Bustamante J, Czerniczyniec A, Cymeryng C, Lores-Arnaiz S (2008) Age related changes from youth to adulthood in rat brain cortex: nitric oxide synthase and mitochondrial respiratory function. Neurochem Res 7:1216–1223
Manczak M, Jung Y, Park BS, Partovi D, Reddy PH (2005) Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem 92:494–504. doi:10.1111/j.1471-4159.2004.02884.x
Li J, O W, Li W, Jiang ZG, Ghanbari HA (2013) Oxidative stress and neurodegenerative disorders. Int J Mol Sci 14:24438–24475. doi:10.3390/ijms141224438
Gandhi S, Abramov AY (2012) Mechanism of oxidative stress in neurodegeneration. Oxidative Med Cell Longev 2012:428010. doi:10.1155/2012/428010
Shimohama S, Tanino H, Kawakami N, Okamura N, Kodama H, Yamaguchi T, Hayakawa T, Nunomura A et al (2000) Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem Biophys Res Commun 273:5–9
De La Monte SM, Wands JR (2006) Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheim Dis 9:167–181
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394
Konsman JP, Kelley K, Dantzer R (1999) Temporal and spatial relationships between lipopolysaccharide-induced expression of Fos, interleukin-1beta and inducible nitric oxide synthase in rat brain. Neuroscience 89:535–548
Marty V, El Hachmane M, Amedee T (2008) Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. Eur J Neurosci 27:3132–3150. doi:10.1111/j.1460-9568.2008.06296.x
Acknowledgements
This work was supported by a grant from the University of Naples Federico II—Ricerca Dip 2015—and by a FIRB-Futuro in Ricerca grant (RBFR12QW4I_004) from the Italian Ministry of Education, University and Research (MIUR). The authors wish to thank Dr. Emilia de Santis for the skillful management of the animal house.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Treatment, housing, and euthanasia of animals met the guidelines set by the Italian Health Ministry. All experimental procedures involving animals were approved by “Comitato Etico-Scientifico per la Sperimentazione Animale” of the University of Naples Federico II.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cigliano, L., Spagnuolo, M.S., Crescenzo, R. et al. Short-Term Fructose Feeding Induces Inflammation and Oxidative Stress in the Hippocampus of Young and Adult Rats. Mol Neurobiol 55, 2869–2883 (2018). https://doi.org/10.1007/s12035-017-0518-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0518-2